)
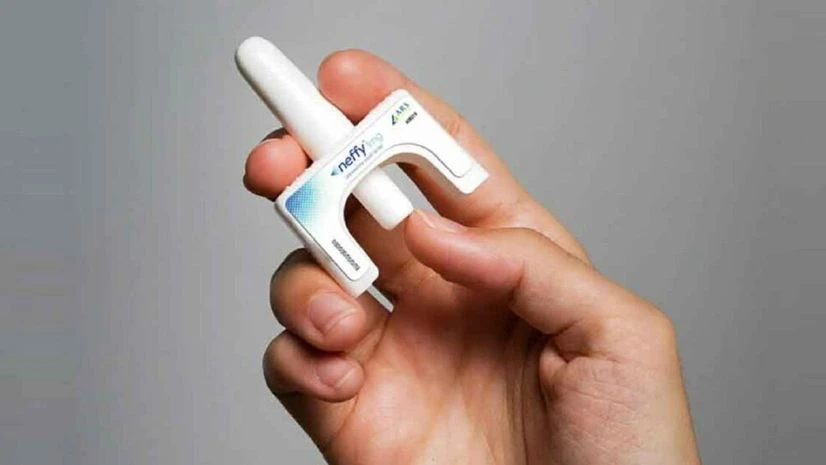
Neffy, a single-dose nasal spray administered into one nostril, was approved for use in adult and pediatric patients who weigh at least 30 kilograms | Credit: X/@US_FDA
The US Food and Drug Administration has approved ARS Pharmaceuticals’ nasal spray as the first needle-free emergency treatment for potentially fatal allergic reactions, the regulator said on Friday.
Shares of ARS Pharmaceuticals rose nearly 9 per cent on the news.
The spray, which will be sold under the brand name neffy, is seen as an alternative to EpiPen and other autoinjectors like Kaleo’s Auvi-Q that are filled with epinephrine, a life-saving drug used by people at risk of anaphylaxis and other allergic reactions.
Anaphylaxis is a severe, life-threatening allergic reaction that typically involves multiple parts of the body and is considered a medical emergency.
Neffy, a single-dose nasal spray administered into one nostril, was approved for use in adult and pediatric patients who weigh at least 30 kilograms.
“Some people, particularly children, may delay or avoid treatment due to fear of injections,” said Kelly Stone, an associate director at the FDA’s Center for Drug Evaluation and Research, adding that the availability of the nasal spray may reduce barriers to rapid treatment.
Neffy’s approval is based on four studies in 175 healthy adults without anaphylaxis that measured the epinephrine concentrations in the blood following administration of neffy or approved epinephrine injection products.
(Only the headline and picture of this report may have been reworked by the Business Standard staff; the rest of the content is auto-generated from a syndicated feed.)
First Published: Aug 09 2024 | 10:39 PM IST





