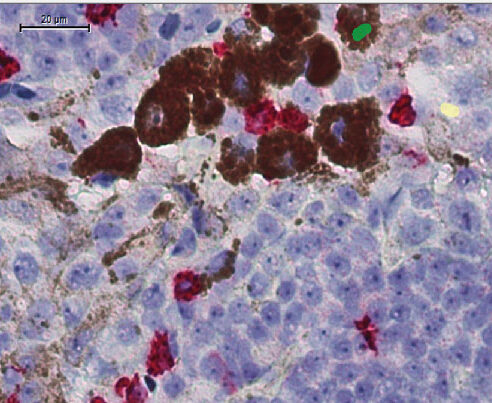
A new study offers clues to overcoming one of the most stubborn challenges in cancer treatment: resistance to immunotherapy. By examining both mouse models and clinical data from melanoma patients, the research team from Memorial Sloan Kettering Cancer Center (MSKCC) and the Earle A. Chiles Research Institute in Oregon has uncovered immune signatures that may help predict who will respond to a new combination immunotherapy—and why.
The research, published in Science Translational Medicine, centers on a combination of anti-PD-1 and anti-LAG-3 antibodies—two types of immune checkpoint inhibitors that aim to rev up the immune system’s attack on tumors. These therapies target proteins used by tumor cells to suppress T-cell activity. When successful, they unleash the immune system’s full anticancer potential. Yet despite their promise, immune checkpoint inhibitors don’t work for everyone. Many patients initially respond to anti-PD-1 drugs like nivolumab, only to become resistant over time.
“Immuno-oncology has given us some of the most powerful treatments in cancer,” said co-senior author Margaret Callahan, MD, PhD, of MSKCC. “But we’re still pretty bad at predicting which patients will respond to which therapies. That’s the real problem we set out to tackle.”
Pinpointing resistance—and a possible solution
To investigate, the researchers used mice engineered to develop tumors that either resisted or responded to anti-PD-1 treatment. In the resistant group, they noticed high levels of immune cells expressing the protein LAG-3. But when these mice were treated with a combination of anti-PD-1 and anti-LAG-3 antibodies, their tumors shrank dramatically.
Digging deeper, the team discovered that this therapeutic combo seemed to reprogram a particular group of immune cells: regulatory T cells (Tregs). Normally, Tregs keep the immune system in check, but in tumors, they often suppress the immune attack. In treated mice, the combination therapy destabilized these Tregs—essentially turning them from peacekeepers into warriors.
“What we saw was a functional reprogramming of the Tregs,” explained Callahan. “They stopped behaving like regulatory cells and started acting more like effector cells, producing inflammatory signals you don’t normally see in Tregs.”
Translating mouse insights to human melanoma
To determine whether the findings held up in human patients, the researchers analyzed blood samples from 117 people with metastatic melanoma who were treated with nivolumab and relatlimab (the anti-LAG-3 antibody). Just as in the mice, patients who developed these “fragile” or reprogrammed Tregs shortly after treatment tended to fare better. They were more likely to respond to therapy—and lived longer.
“That was the moment it clicked,” said Callahan. “We saw this weird population of Tregs emerging in some patients, and it matched exactly what the mouse model had shown. That’s when we knew we were looking at two sides of the same coin.”
Importantly, the appearance of these unusual Tregs—those expressing markers not typically associated with regulation but rather with immune activation—was a strong indicator of treatment success. Patients whose Tregs shifted toward this fragile or effector-like phenotype were more likely to benefit from the combination therapy.
Implications for clinical decision-making
The new biomarker isn’t perfect. Because it only appears after treatment begins, it can’t be used to decide whether to start the therapy in the first place. But it does offer an early readout—weeks before imaging scans would typically show a response—on whether the treatment is working.
“It’s what we call a pharmacodynamic biomarker,” said Callahan. “It doesn’t predict response up front, but it gives you an early signal once treatment starts. That’s still incredibly valuable because it lets us adapt if we’re not seeing the changes we hope for.”
This insight could change how oncologists manage patients who fail first-line therapies. Currently, when patients become resistant to anti-PD-1 monotherapy, treatment decisions are often a shot in the dark. But if clinicians can detect this reprogramming of Tregs early on, they may have a clearer sense of whether the PD-1 + LAG-3 combo—often referred to as “nivo/rella”—is worth continuing or whether an alternative strategy is needed.
“Right now, we’re fishing in the dark after PD-1 failure,” said Callahan. “But if we could validate this biomarker in larger, prospective trials, it might give us a way to be more intentional—more precise—about how we use the tools we already have.”
Nivolumab combined with relatlimab has become an increasingly popular option for melanoma patients, offering a middle ground between the relatively gentle PD-1 monotherapy and the highly potent—but highly toxic—combination of PD-1 and CTLA-4 inhibitors.
“Nivo/rella seems to be becoming the go-to frontline option for many oncologists,” Callahan noted. “It’s better tolerated than CTLA-4 combos but offers a stronger response than PD-1 alone. That’s why understanding who is most likely to benefit from it is so important.”
And the implications may extend beyond melanoma. If this kind of Treg plasticity proves to be a general mechanism of resistance reversal, it could inform combination immunotherapy approaches across a range of tumor types.
The team hopes to validate their findings in a prospective study, where patients are monitored in real-time as they begin treatment. Such a study could help determine how best to integrate this biomarker into clinical workflows.







![Best Weight Loss Supplements [2022-23] New Reports!](https://technologytangle.com/wp-content/uploads/2022/12/p1-1170962-1670840878.png)




