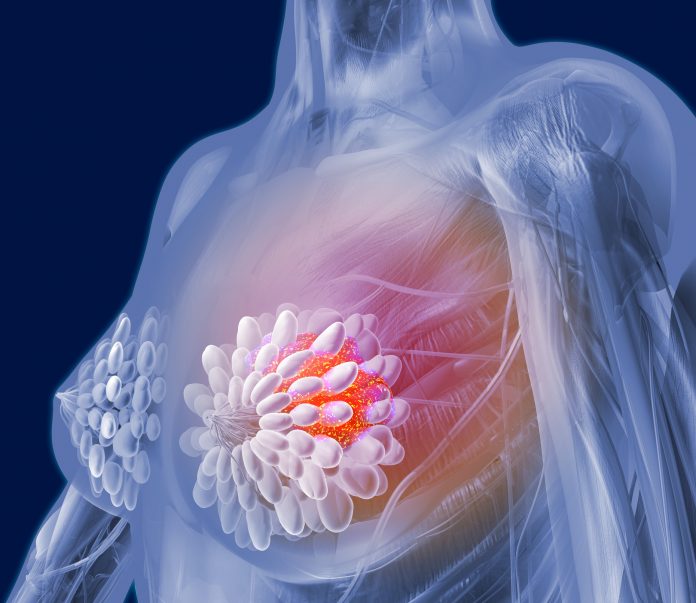
Researchers at Weill Cornell Medicine have uncovered how omega-6 linoleic acid, a common fatty acid found in seed oils and animal products, plays a significant role in promoting the growth of aggressive triple-negative breast cancer (TNBC). The study, published in Science, reveals a new mechanism through which omega-6 linoleic acid activates a major cancer growth pathway by binding to a protein called FABP5, which is particularly abundant in TNBC, but not other breast cancer subtypes.
“This discovery helps clarify the relationship between dietary fats and cancer, and sheds light on how to define which patients might benefit the most from specific nutritional recommendations in a personalized manner,” said senior author John Blenis, PhD, a professor of cancer research at Weill Cornell Medicine.
Omega-6 fatty acids, including linoleic acid, are essential nutrients that support bodily functions but have been linked to an increased risk of certain cancers, including breast cancer. These fatty acids are especially prevalent in Western diets that are high in processed foods and seed oils. Prior research on omega-6’s impact on cancer has produced mixed results, but this new study is the first to identify a specific biological mechanism linking omega-6 linoleic acid to cancer growth.
In their research, the team focused on TNBC, an aggressive subtype of breast cancer that lacks estrogen, progesterone, and HER2 receptors and is highly resistant to current treatments. The investigators found that linoleic acid activates the mTORC1 pathway, an important regulator of cell growth and metabolism. This activation occurs because the fatty acid forms a complex with FABP5, which is produced at high levels in TNBC, but not in other breast cancer subtypes.
“By binding to FABP5, linoleic acid forms a complex that activates mTORC1, which drives tumor cell growth,” the researchers wrote. “Feeding mice a high linoleic acid diet increased FABP5 levels, mTORC1 activation, and tumor growth.”
The team used several preclinical models, including breast cancer cell lines and patient-derived xenografts (PDX) to confirm team that linoleic acid activated the mTORC1 pathway in triple-negative cells, but not in other hormone-sensitive subtypes. They also found that newly diagnosed TNBC patients had high levels of FABP5 in both their tumors and in blood samples.
The findings could have significant implications for clinical care. The researchers said that FABP5 may serve as a useful biomarker for identifying TNBC patients who could benefit from nutritional or pharmacological interventions targeting the omega-6-mTORC1 signaling pathway. “Future nutritional studies might consider stratifying patients on the basis of FABP5 expression and triple-negative status,” the researchers wrote. In addition, the study suggests that dietary adjustments, aimed at reducing omega-6 intake, could potentially help manage or slow the progression of TNBC in some patients.
The researchers have only begun to explore whether omega-6 fatty acids may play a role in health conditions. Preliminary findings suggest that the FABP5-mTORC1 signaling pathway may also influence the growth of some prostate cancer subtypes. “There may be a broader role for FABP5-mTORC1 signaling in other cancer types and even in common chronic diseases such as obesity and diabetes,” said first author Nikos Koundouros, PhD, a postdoctoral researcher in the Blenis lab.






![Best Weight Loss Supplements [2022-23] New Reports!](https://technologytangle.com/wp-content/uploads/2022/12/p1-1170962-1670840878.png)




