Trace Neuroscience just launched with a $101 million Series A financing led by Third Rock Ventures. The company develops genomic therapies to restore the UNC13A protein’s healthy communication between nerves and muscle cells impacted by neurodegenerative diseases. Its lead program is an antisense oligonucleotide (ASO) designed to treat amyotrophic lateral sclerosis (ALS), including cases of the sporadic form that affects nine out of 10 people with the disease.
They also aim to treat frontotemporal dementia and over half of Alzheimer’s disease patients where UNC13A is lost.
“Genomic-based therapies have begun to transform the lives of people living with ALS. But so far, they have only been effective for those rare forms of the disease caused by SOD1 or FUS mutations, which account for only 3% of all ALS cases. The remaining people, including those with sporadic disease, the most common form that occurs randomly without a clear cause, need new treatments grounded in human genetics with defined mechanisms of action,” said Trace co-founder Aaron Gilter, PhD, professor of genetics at Stanford University.
“UNC13A is a highly compelling genetic target directly linked to ALS disease progression and survival. Insights from the human genome have led to transformative medicines for many diseases, and with what we now know about the role of UNC13A, we believe the time is right to apply this approach to ALS,” said Eric Green, MD, PhD, co-founder and CEO of Trace.
He added, “We envision a world where UNC13A restoration improves outcomes across a range of neurodegenerative diseases, including for the approximately 30,000 people in the U.S. living with ALS.”
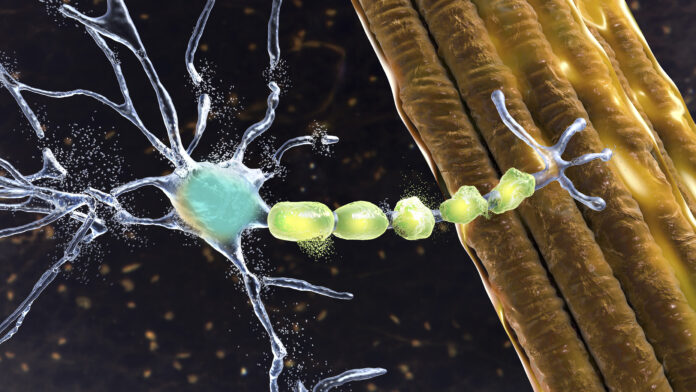
Trace’s formation grew from convergent discoveries by the company’s co-founders linking abnormal function of TDP-43 protein with the loss of UNC13A protein, an essential component for neuronal communication in the brain and spinal cord. In almost all people with ALS, this relationship progressively breaks down and leads to decreased mobility, paralysis and difficulty breathing.
Gitler said, “Our insights into UNC13A biology may be the key to slowing disease progression, preserving or restoring muscle function and extending survival for people living with ALS.”
Approximately 97% of people with ALS produce insufficient amounts of UNC13A, which is directly regulated by the TDP-43 protein that controls RNA splicing. When TDP-43 stops functioning normally, as occurs in nearly all people with ALS, the UNC13A messenger RNA (mRNA) is improperly spliced, which hinders adequate UNC13A production. Trace Neuroscience’s ASO development candidate is designed to deliver a targeted intervention by binding directly to UNC13A mRNA to regulate its processing and ensure proper splicing, thereby correcting synaptic dysfunction and preserving neuronal signaling.
“UNC13A is critical for neurons to communicate amongst each other and with muscles through synaptic function, which is lost in ALS. Being able to re-establish this is groundbreaking. Our focus is now on rapidly translating this science into a life-changing medicine by advancing our lead program toward the clinic,” said co-founder Pietro Fratta, MD, PhD, professor of cellular and molecular neuroscience at the University College London and Francis Crick Institute.





