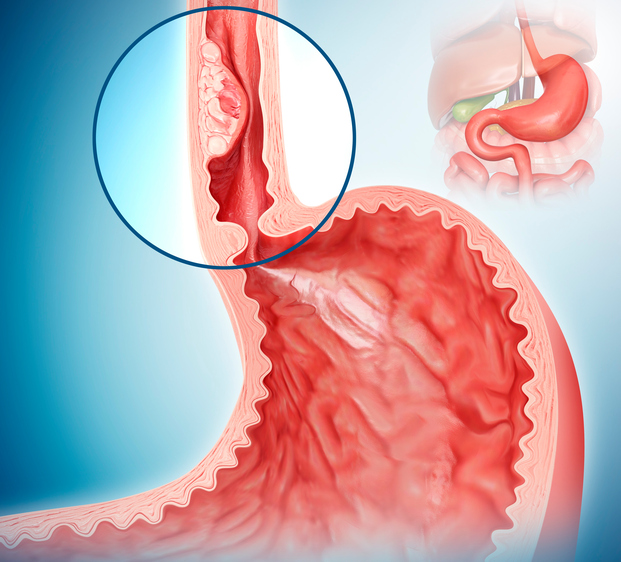
A novel test developed by researchers at the Johns Hopkins Kimmel Cancer Center could help predict which patients with Barrett’s esophagus are most likely to progress to esophageal cancer.
The test, called Esopredict, uses polymerase chain reaction on biopsy samples to measure the level of DNA methylation changes in the genes RUNX3, p16, HPP1, and FBN1. Methylation changes often occur early during abnormal cell growth, before conditions can be detected clinically.
RUNX3, p16, and HPP1 have been extensively validated in several previous studies of risk prediction in Barrett’s esophagus patients, while FBN1 was discovered in a genome-wide study of methylation markers in patients with esophageal adenocarcinoma, explained study senior author Stephen Meltzer, MD, American Cancer Society Clinical Research professor, and The Harry & Betty Myerberg/Thomas R. Hendrix Professor of Gastroenterology at the Johns Hopkins University School of Medicine & Sidney Kimmel Comprehensive Cancer Center.
By combining the Esopredict results with patients’ age, physicians can predict the likelihood that patients with Barrett’s esophagus—a premalignant condition in which parts of the esophagus become damaged by chronic acid reflux—will advance to high-grade dysplasia or esophageal cancer. This then helps them determine how often to monitor or manage patients with potential neoplastic progression.
“Current risk prediction in patients with Barrett’s esophagus relies on histologic findings and grading of dysplasia,” said Meltzer. “The majority (90%) of patients do not have dysplasia but the 7% who do have indefinite or low-grade dysplasia are plagued by high interobserver disagreement among pathologists, as well as unclear management options.”
Patients with Barrett’s esophagus typically undergo esophagogastroduodenoscopy surveillance every three to five years to determine the level of dysplasia (none, indefinite, low-grade, or high-grade). Those with high-grade dysplasia or esophageal cancer can be treated with endoscopic eradication therapies such as radiofrequency ablation or endoscopic resection that have high success rates.
However, up to 25% of patients progress to high-grade dysplasia or esophageal cancer between surveillance appointments, representing missed opportunities for early intervention. Furthermore, esophageal cancer is the second-most lethal cancer in the U.S., with a five-year survival of only 21%.
Meltzer and his team developed Esopredict to see if they could better identify patients at risk for progression.
They validated the tool in previously collected biopsies from 240 patients with Barrett’s esophagus from six medical centers and report in The American Journal of Gastroenterology that the overall risk for neoplastic progression was 5.1%.
When the researchers stratified the samples into lower- and higher-risk groups based on Esopredict findings, they found that patients in the higher risk group were 6.4 times more likely to progress to high-grade dysplasia or esophageal cancer within five years than those in the lower risk group.
Further stratification into low-, low moderate-, high moderate-, and high-risk categories revealed that the likelihood of progression was 15.2 times higher for patients categorized and high risk versus those in the low-risk category.
“By further stratifying into four risk levels, we gain deeper insights for surveillance and treatment considerations,” the researchers remark. Just 1.9% of patients in the low-risk group experienced progression, compared with 4.5% of those with low moderate-risk, 8.1% of those with high-moderate risk and 21.5% of patients with high-risk Esopredict scores.
Meltzer and his colleagues also note that nine of 110 patients in the initial validation data set had an outcome biopsy showing progression less than one year after their index biopsy. Of these, six (66.7%) were predicted in the higher-risk category.
In addition, 14 (57.7%) of 26 patients with no dysplasia who progressed between years zero and five would have been classified by Esopredict into the higher-risk category. These would likely be overlooked based on existing standard-of-care methods, say the authors.
“The low cost, high throughput, and repeatably consistent performance of the biomarkers across multiple development studies make Esopredict an ideal technology for [Barrett’s esophagus] surveillance,” Meltzer et al. conclude.
The test is now commercially available in the United States and is marketed by Previse as Meltzer and his team continue to test its performance. They are currently “profiling multiple anatomic locations within a single endoscopy, as well as multiple endoscopic timepoints within the same patient, to observe trends in these test results when measured across space and time,” he says. “We are also carrying out further studies in Barrett’s esophagus patients without any dysplasia.”








![Best Weight Loss Supplements [2022-23] New Reports!](https://technologytangle.com/wp-content/uploads/2022/12/p1-1170962-1670840878.png)




