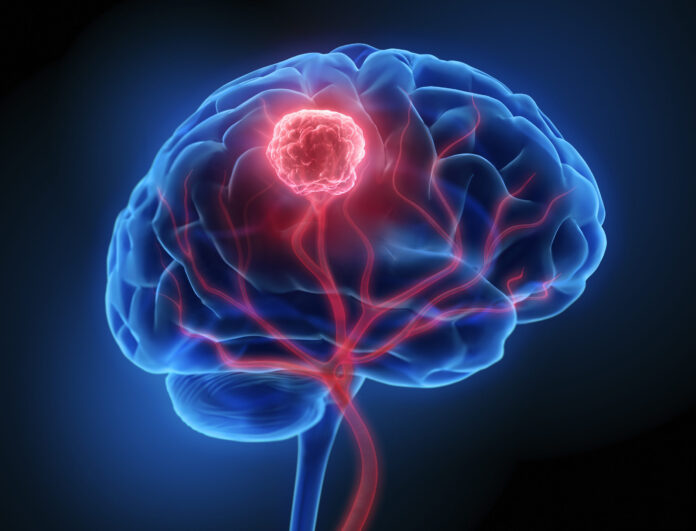
A new type of stem cell has been found in the brain that could lead to the development of better treatments in adult glioblastoma (GBM), one of the deadliest types of brain tumors. The team’s finding could help explain how adult brain cells use developmental processes to form tumors like glioblastoma. They also created a disease-risk map, highlighting enriched risk-associated with autism spectrum disorder in second-trimester intratelencephalic neurons.
“Many brain cancers are thought to hijack developmental programs and to originate from ‘cancer stem cells’ that resemble embryonic cell types, so a similar approach to ours could help identify the cells of origin. Many brain cancers are thought to hijack developmental programs and to originate from ‘cancer stem cells’ that resemble embryonic cell types, so a similar approach to ours could help identify the cells of origin,” senior author Arnold R. professor of neurology at the University of California, San Francisco (UCSF) and co-corresponding author of the paper told Inside Precision Medicine.
The study appears in Nature and is led by Li Wang, a postdoctoral scholar in Arnold Kriegstein’s lab at UCSF.
“Tumors are hard to detect early because by the time they produce symptoms they have already grown and spread. GBM readily invades normal brain tissue and is impossible to fully remove surgically. Despite all known treatments the tumors invariably recur,” Kriegstein added.
The researchers conducted a broad genomic survey of human brain cells taken from corpses from the first two decades of life. They obtained these from the National Institutes of Health’s (NIH) NeuroBioBank, and local hospitals associated with UCSF. The samples were donated from individuals from early life through adolescence. They were then analyzed for gene expression in thousands of individual cells.
They started with paired single-nucleus chromatin accessibility and transcriptome data from 38 human neocortical samples encompassing both the prefrontal cortex and the primary visual cortex. These samples span five main developmental stages, ranging from the first trimester to adolescence. In parallel, we performed spatial transcriptomic analysis on a subset of the samples to illustrate spatial organization and intercellular communication.
Moreover, combining single-cell profiling, progenitor purification and lineage-tracing experiments, they “untangled” the lineage relationships among progenitor subtypes during the neurogenesis-to-gliogenesis transition. They identified a tripotential intermediate progenitor subtype—tripotential intermediate progenitor cells (Tri-IPCs). Tri-IPC is responsible for the local production of GABAergic neurons, oligodendrocyte precursor cells and astrocytes.
“Many brain diseases begin during different stages of development, but until now, we haven’t had a comprehensive roadmap for simply understanding healthy brain development,” said Kriegstein. “Our map highlights the genetic programs behind the growth of the human brain that go awry during specific forms of brain dysfunction,” he continued.
The researchers then analyzed which parts of each chromosome were available for expressing genes in each cell and labeled where each cell had been taken from in the brain—focusing on cells taken from the front and the back of the cerebral cortex, regions that in humans are responsible for learning, memory, and language.
The researchers found a particular stem cell in the young brain that is capable of forming the cells found in tumors. They identified a group of stem cells that had begun to express genes normally found across three mature cell types. Many stem cells in the developing brain mature into just one cell type such as a neuron or a support cell. Some can mature into two types, but these stem cells could mature into three lineages: two types of support cells known as glia, and one type of neuron.
The researchers thought this ability might enable it to give rise later in life to glioblastoma, which contains three similar cell types.
In addition, by integrating their atlas data with large-scale genome-wide association study data, they created a disease-risk map highlighting enriched risk associated with autism spectrum disorder in second-trimester intratelencephalic neurons.








![Best Weight Loss Supplements [2022-23] New Reports!](https://technologytangle.com/wp-content/uploads/2022/12/p1-1170962-1670840878.png)




