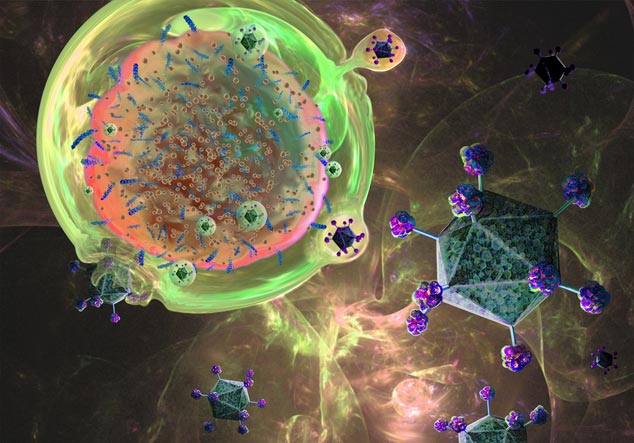
In a new study published in Science Advances, researchers at the Baylor College of Medicine explore the cellular mechanics behind CAR T cell therapies and their varying efficacy in treating different types of cancers. While CAR T cells have revolutionized treatment for blood cancers like leukemia, their success in solid tumors has been limited. The team’s research sheds new light on the molecular interactions that drive these therapies, offering hope for improved designs in the future.
“We wanted to understand the distinct mechanisms that make some CAR T cells effective in certain cancers but less so in others,” explains senior author Nabil Ahmed, professor of pediatrics—hematology and oncology at Baylor and Texas Children’s. “By delving into what happens at the molecular level, we can potentially design a new generation of CAR T cells better suited for solid tumors.”
The dual nature of CAR T Cells
CAR T cells, or chimeric antigen receptor T cells, are genetically engineered immune cells programmed to target and destroy cancer. Two main CAR T cell designs dominate the clinical landscape, each with unique strengths and limitations. Almost all clinically tested CARs use costimulatory domains from CD28ζ or 4-1BB.
Ahmed explains that the 28ζ CAR T cells acts like a muscle car, offering rapid, high-intensity responses. However, their energy-intensive approach often results in short-lived effectiveness. Conversely, the 4-1BB CAR T cells are akin to a fuel-efficient vehicle, delivering slower but more sustainable performance. This dichotomy is well-documented in leukemia treatments but poorly understood in the context of solid tumors.
“Solid tumors have proven to be a much tougher challenge,” Ahmed noted. “In trials, we see response rates barely reaching 25%, and even those are rarely durable. We needed to understand why.”
A “battle of rafts” at the molecular level
The researchers focused on lipid rafts, specialized microdomains on the surface of T cells. These rafts serve as platforms where crucial interactions between CAR T cells and tumors occur. First author Ahmed Gad, postdoctoral associate in Ahmed’s lab, who pioneered the study’s experimental design, described the importance of these rafts.
“Cell membranes are highly fluid, making stable interactions difficult,” said Gad. “Lipid rafts act like islands, anchoring receptors and enabling them to form strong, lasting connections with tumor cells.”
The team studied how CAR T cells interact with tumors at different time points. They found that 28ζ CAR T cells have a transient relationship with lipid rafts, quickly engaging and disengaging from tumor cells. This behavior facilitates their “serial killing” capability, allowing them to attack multiple targets in rapid succession.
In contrast, 4-1BB CAR T cells form longer-lasting interactions. They accumulate adhesion molecules and signaling proteins in their lipid rafts, creating highly-stable synapses with tumor cells. These prolonged engagements likely explain their superior long-term effectiveness, especially in leukemia.
Structural vs. functional avidity
One of the study’s key insights was the distinction between two types of “avidity” in CAR T cell interactions: structural and functional.
“Structural avidity reflects the physical bond between a T cell and a tumor cell,” Gad explained. “Functional avidity, on the other hand, measures how this bond translates into effective killing.”
The 28ζ CAR T cells excel in functional avidity. They rapidly direct toxic vesicles, known as lytic granules, to the synapse, destroying the tumor cell before moving on to the next target. In contrast, 4-1BB CAR T cells exhibit high-structural avidity, forming mechanically robust synapses that allow for sustained interaction and repeated signaling.
“These findings are a huge step forward,” said Ahmed. “By understanding the nuances of structural and functional avidity, we can start to design CAR T cells that combine the best of both worlds.”
Implications for solid tumor therapies
The team believes their findings could guide the development of hybrid CAR T cells tailored for solid tumors. By optimizing the balance between rapid action and durability, these next-generation therapies might overcome the challenges posed by solid tumor environments.
“We’re essentially learning from the successes and failures of current designs,” Ahmed said. “It’s like reverse-engineering the perfect vehicle by studying both a race car and a reliable family sedan.”
The study also highlighted the need for deeper investigation into the tumor microenvironment. Solid tumors often create immunosuppressive conditions, luring T cells into a trap where they become co-opted rather than killing the tumor. The researchers hope to identify strategies to counteract these effects.
“For years, we’ve been treating CAR T cells as black boxes—effective, but mysterious,” Ahmed concluded. “Now, we’re starting to see what’s happening inside. It’s a revelation.”








![Best Weight Loss Supplements [2022-23] New Reports!](https://technologytangle.com/wp-content/uploads/2022/12/p1-1170962-1670840878.png)




