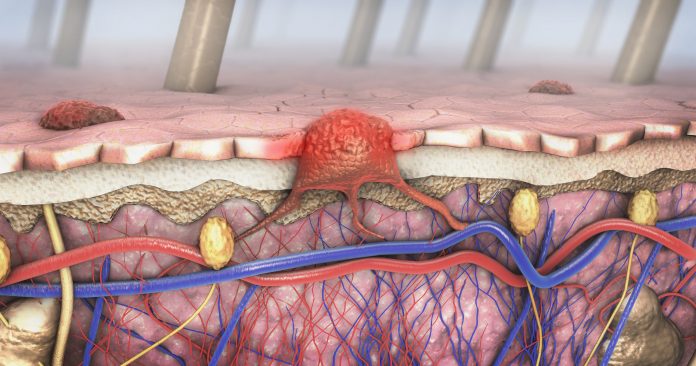
New research led by scientists at the Wistar Institute has identified a potential new therapeutic strategy to address treatment-resistant melanoma by targeting the S6K2 gene. The research, published in Science Translational Medicine, provides a fertile path for the development of treatments targeting S6K2 to address the 30% of melanoma patients who develop resistance to treatments due to mutations in the NRAS gene, called NRASMUT melanoma.
“This work shows that, even in the face of notoriously treatment-resistant melanoma, targeting S6K2 is a viable strategy for improving therapeutic outcomes,” said study lead Jessie Villanueva, PhD, an associate professor at Wistar’s Ellen and Ronald Caplan Cancer Center.
Melanoma incidence has been increasing for more than 20 years, rising from 18 to 24 cases per 100,000 people per year in the United States. This increase has been particularly notable in people under the age of 30. Despite advances, the disease remains difficult to treat. A class of drugs known as MAPK inhibitors, a standard treatment approach, have shown limited success, with resistance emerging in approximately 80% of cases.
The Wistar Institute’s research demonstrated the potential of targeting the S6K2 gene, which they demonstrated is linked to the survival of NRASMUT melanoma cells that are resistant to MAPK inhibitors. An analysis of patient data revealed a strong correlation between high expression of S6K2 and poor patient outcomes in those with NRASMUT melanoma. Further experiments in the lab showed that silencing the S6K2 gene in melanoma cell lines led to the death of cells that had previously been resistant to MAPK inhibition.
The researchers also identified a crucial link between S6K2 and lipid metabolism, which plays a key role in the survival of these resistant melanoma cells. “We were able to target lipid homeostasis, a vital metabolic pathway in cancer cells, and induce cell death in melanoma cells resistant to MAPK inhibition,” said Brittany Lipchick, PhD, associate staff scientist in the Villanueva lab and co-first author of the study. “Our findings suggest a clear path forward for more preclinical research on these treatment options. Not only did our treatments work in the lab, but they also appear to be quite safe.”
In addition to directly targeting S6K2, the researchers also identified a potential combination therapy using fenofibrate, which activates PPARα, and DHA, also known as omega-3 fatty acids.
“Since there are no commercially available inhibitors of S6K2, we used Fenofibrate (which activates PPAR-alpha) and DHA (a source of polyunsaturated fatty acids) to mimic the effects of S6K2 depletion,” Villanueva told Inside Precision Medicine. “The advantage of these compounds is that they are already used in humans, making this approach potentially translatable to clinical studies.”
NRASMUT melanoma is notoriously difficult to treat due to the persistence of oncogenic NRAS mutations, which continuously activate the MAPK pathway, a key driver of melanoma development. Previous efforts to inhibit the MAPK pathway in NRASMUT melanoma have largely failed, with single-agent MAPK inhibitors proving to be only minimally effective. In combination therapies, inhibiting both the MAPK and PI3K pathways has resulted in toxicities that limit their clinical use.
“We knew that certain treatments could theoretically work against melanomas that resist treatment with MAPK inhibitors, but they were a non-starter because they were incredibly toxic,” said Adam Guterres, PhD, associate staff scientist and co-first author. “Our work shows that we can still fight this stubborn melanoma without a prohibitively toxic treatment.”
The Wistar team hypothesized that targeting a specific downstream subnetwork, rather than directly inhibiting the MAPK or PI3K pathways, might avoid these toxicities and offer a more effective therapeutic approach.
Pursuing this hypothesis, the researchers focused on the S6 kinase (S6K) family, particularly S6K2, an enzyme that plays an important role in cellular metabolism and survival. Previous studies have shown that S6K1 is implicated in the development of drug resistance in melanoma, but the role of S6K2 is still not completely understood. By mapping the signaling networks downstream of the MAPK and PI3K pathways, the investigators discovered an S6K2/PPARα subnetwork critical for melanoma cell survival, particularly in cases resistant to MAPK inhibitors.
The investigators demonstrated that selectively depleting S6K2 in melanoma cells triggered a cascade of events leading to lipid metabolic imbalance and endoplasmic reticulum (ER) stress, which resulted in oxidative cell death. Notably, this effect was observed in NRASMUT melanoma cells resistant to MAPK inhibition (MAPKi), but not in MAPK-sensitive cells. “Our findings underscore that MAPKi-resistant and -sensitive cells have different signaling dependencies both upstream and downstream of mTORC1,” the researchers wrote.
The team’s research shows that NRASMUT melanoma cells are vulnerable due to their dependence on a specific lipid metabolic pathway that can be disrupted by targeting S6K2. “Selective targeting of S6K2 or its effectors represents a viable opportunity for therapeutic intervention in S6K2-dependent tumors such as MAPKi-R NRASMUT melanoma,” the researchers wrote. Importantly, they noted that inhibition of S6K2 does not cause the same toxicities as broader inhibition of the mTORC1/S6K1 axis, which has been linked to adverse side effects in preclinical models and clinical trials.
The researchers plan further preclinical studies to test the combination of FNB and DHA in animal models of melanoma and will search for other potential inhibitors of S6K2. In addition, they plan to investigate the immunomodulatory effects of FNB and DHA, which could provide important insights into their potential to improve the efficacy of immune-based therapies.








![Best Weight Loss Supplements [2022-23] New Reports!](https://technologytangle.com/wp-content/uploads/2022/12/p1-1170962-1670840878.png)




