Researchers have sequenced the genomes of all established bacterial species that cause Lyme disease, with the hope that this may help create more effective treatments for the tick-borne infection.
Investigators genetically mapped 47 spirochete bacteria strains in the Borrelia burgdorferi sensu lato clade that are responsible for Lyme disease, comprising 23 species.
In so doing, they have discovered the evolutionary path of the pathogen, how it spread and how it has maintained its virulence.
The findings, published in the journal mBio, could improve the diagnosis, treatment and prevention of Lyme’s disease, which is the most prevalent and rapidly expanding tick-borne infection in Europe and North America.
“By understanding how these bacteria evolve and exchange genetic material, we’re better equipped to predict and respond to changes in their behavior, including potential shifts in their ability to cause disease in humans,” explained senior author Weigang Qiu, PhD, a biology professor at City University of New York.
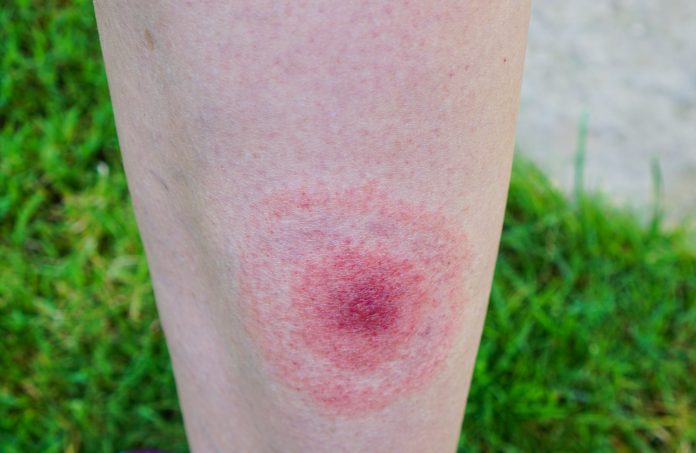
Lyme disease can begin with a characteristic circular or oval-shaped rash around a tick bite and may cause fever, headache, fatigue and joint pain.
Left untreated, the infection may progress and cause serious complications, spreading to the joints, heart and nervous system.
Genome comparisons between and within species of disease-causing bacteria are essential to reconstruct their evolutionary origins and track epidemiological spread, say the researchers.
These studies also allow molecular mechanisms of pathogenicity to be identified and aid the design molecular and ecological approaches to disease prevention, diagnosis, and treatment.
The team reported the complete genetic sequences of 47 isolates that encompass the 23 established species of Borrelia bacteria that cause Lyme disease.
The analysis revealed a complete lack of horizontally transferred DNA from other bacterial genera, suggesting a co-adapted gene pool reflective of an obligatory association between the tick and host.
Different species shared similar, though not identical, plasmids, and the maintenance of these throughout Borrelia divergence indicated these were a key adaptive feature of this genus.
Phylogenetic reconstruction of Borrelia genomes revealed the original divergence of Eurasian and North American lineages.
It also outlined subsequent dispersals that introduced B. garinii, B. bavariensis, B. lusitaniae, B. valaisiana, and B. afzelii from East Asia to Europe and B. burgdorferi and B. finlandensis from North America to Europe.
The plasmid types and their gene families indicated that the ancestral population likely originated an estimated 55–180 million years ago.
This was before the break-up of the continents, when the supercontinent Pangea still existed, and helps explain the widespread distribution of the disease.
Natural selection and recombination within and between species aided the evolution of adaptive sequences at host-interacting lipoprotein loci and contributed to human virulence, despite the genome-wide clonal structure of its natural populations.
The findings provide a framework for similar work with other infectious diseases caused by pathogens.
“This comprehensive, high-quality sequencing investigation of Lyme disease and related bacteria provides the foundation to propel the field forward,” said co-author Steven Schutzer, MD, a professor at Rutgers New Jersey Medical School professor whose research is focused on the interface between the immune system and microbes.
“Every modern research project—from clinical to public health to ecology and evolution to bacterial physiology to medical-tool development to host-bacteria interaction—will benefit from this work.”
The investigators have created a web-based software tool available at the BorreliaBase.org so others can compare Borrelia genomes and research the ability of these bacteria to infect humans.





