U.S. researchers have shone further light into the precise way that CRISPR/Cas9 gene editing actually works and circumvents unintended off-target effects, which could lead to more accurate cellular therapies.
The preclinical research, published in the journal Cell Chemical Biology, outlines several steps that are necessary for the Cas9 complex to become active and allow it to accurately edit genes.
Specifically, the findings reveal a molecular mechanism by which on- and off-target DNA sequences are identified relative to the guide RNA template.
The study could improve the design of CRISPR-based gene therapies and aid their delivery directly within the body rather than through the laborious engineering of cells ex vivo.
“It’s much easier to deliver the pre-programmed Cas9 complex in the form of a lipid nanoparticle to directly edit target cells in the patient when we can be sure that it’s going to do its job correctly,” explained senior researcher Nikolaos Sgourakis, PhD, an associate professor at The Children’s Hospital of Philadelphia center for computational and genomic medicine.
“For example, precise gene editing using CRISPR technology could be used to directly modify T cells in patients, and that would allow us to simplify the adaptation of CAR-Ts and other cellular therapies.”
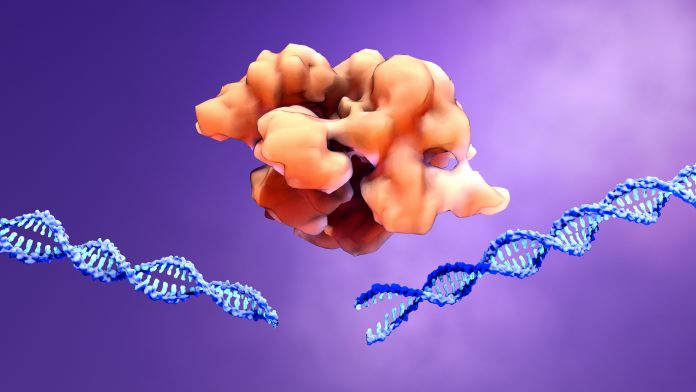
CRISPR/Cas9 has been adapted from naturally occurring gene editing systems that bacteria use against viruses.
The technology has revolutionized genome editing and acts as molecular scissors, cutting into DNA to add or remove sequences at sections, with a guide RNA directing it to the correct part of the genome.
However, the Cas9 enzyme can also create unintended DNA breaks, which could be harmful or turn cells cancerous.
To understand its mechanism of action better, researchers used nuclear magnetic resonance spectroscopy to visualize how the Cas9 complex moved from an inactive to an active state through a dynamic conformational switch.
They found that a high-fidelity Cas9 variant increased the reliability of DNA recognition by stabilizing an intermediate surveillance state.
The intermediate, surveillance structure acted as a proofreader that regulated Cas9 cutting activity and helped discriminate between on- and off-target DNA sequences.
Specifically, a discrete protein conformational state enabled recognition of mismatched DNA sequences that shifted Cas9 away from its active form.
The findings establish a system in which there is molecular recognition through dynamic sensing that is enabled by a protein-based switch.
“We always had a suspicion that what we could see from existing structures from the gene editing process was not telling us the whole story,” said Sgourakis.
His team’s insights into the underlying molecular mechanisms affecting Cas9 activity could improve the effectiveness of gene editing technology and other cell therapies such as CAR-T immunotherapy.





