By Prathiba Raju and Abhijeet Singh
Mumbai: After creating a buzz in medical circles with claims that its eye drop ‘PresVu Eye’ could potentially replace reading glasses for those suffering from presbyopia, the promising story of Entod Pharmaceuticals seems to be heading towards an anti-climactic end.
The controversy arose after the company made certain claims about its drug that were not in line with the regulatory approval. The DCGI suspended the approval for the newly launched drug, prohibiting the company from manufacturing and marketing it. The regulator stated that the company tried to justify claims for which approval had not been granted.
An official communiqué issued by the Directorate General of Health Services (DGHS) and the Central Drugs Standard Control Organization (CDSCO) stated, “The company made claims for the drug product for which it had not obtained approval from the Central Licensing Authority, thereby violating provisions under the New Drugs and Clinical Trial Rules, 2019.”
In response, Nikkhil K. Masurkar, CEO of Entod Pharmaceuticals, asserted, “The suspension order from the DCGI does not reference any specific violation of the Drugs and Cosmetics Act for this action.”
Regarding the company’s next steps, he stated, “We have decided to challenge this suspension in court to seek justice.”
Addressing the reasons cited by the domestic drug regulator, the CEO further added, “Entod Pharmaceuticals has not made any unethical or false presentations of facts to the media or public concerning PresVu Eye Drops. All information disclosed to the media was based on the recent DCGI approval for the treatment of presbyopia in adults and the results of our phase 3 clinical trial conducted in India.”
Defending the drug, Masurkar elaborated in a statement, “Our approval by the DCGI was based on a valid controlled clinical trial in 234 patients, which successfully demonstrated the efficacy and safety of these eye drops in presbyopia patients, as measured using Snellen’s chart—a standard for assessing near vision improvement.”
Masurkar also pointed out, “Similar eye drops with the same active ingredient and concentration have been approved by the US FDA and marketed in the US for the past three years without any serious complications. The FDA has taken no action against companies marketing the same product in the USA.”
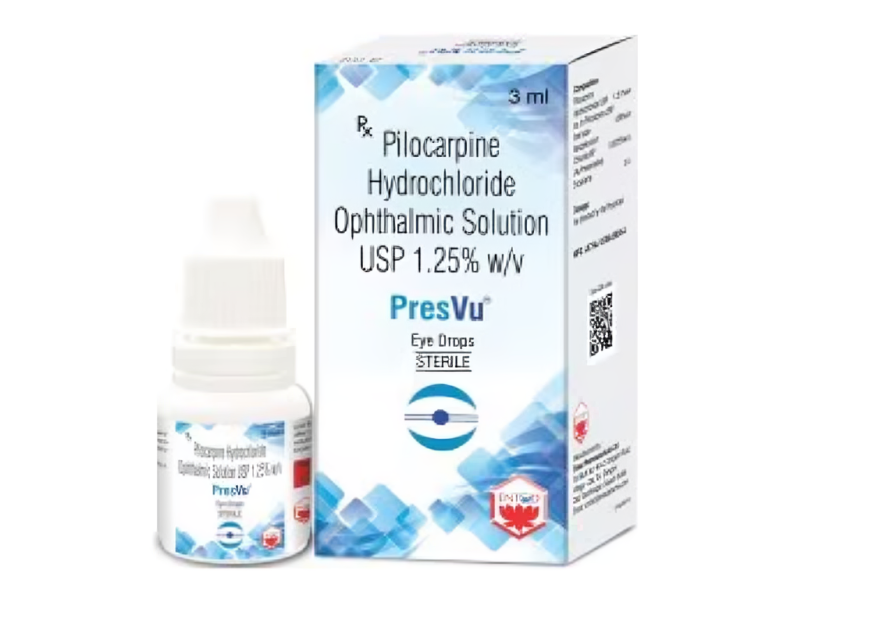
Last month, the DCGI, under the CDSCO, had granted approval to manufacture and market Pilocarpine Hydrochloride Ophthalmic Solution USP 1.25% w/v for the treatment of presbyopia in adults. However, following several media reports, the DCGI issued a notice directing the company to justify claims of being “the first eye drop in India designed to reduce the need for reading glasses.”
Notably, several top officials within the company had previously told news agency ANI that there had been “unethical and false presentations of facts by media” regarding PresVu Eye Drops. These claims were later denied by the company noting the issue has been made sensational by the media.