The top-line results last September from Eisai’s Phase 3 trial of the anti-amyloid antibody lecanemab galvanized the field, but scientists said they needed to see the data before passing judgement. Now they have. At the 15th Clinical Trials on Alzheimer’s Disease conference, held November 29 to December 2 in San Francisco and online, scientists presented detailed findings to a standing-room-only audience of some 2,000 people and many more on livestream. Four speakers reported that secondary and biomarker measures were consistent, and of similar magnitude to the effect on primary. Overall, lecanemab appeared to slow disease progression by about one-quarter, and caused the brain edema known as ARIA-E in one of eight participants. The data were published in the New England Journal of Medicine November 29, the same day as the presentation.
- On lecanemab, all clinical outcomes showed similar slowing of decline.
- Amyloid plaque dropped below 25 centiloids after 18 months.
- AD biomarkers fell; neurodegeneration markers were mixed.
The audience responded positively. Many scientists praised the trial’s execution, and expressed relief that the presentations appeared thorough and transparent. “The Clarity trial is a landmark in AD therapeutic research, the culmination of over three decades of efforts across the field,” said Paul Aisen of the University of Southern California in San Diego. Randall Bateman of Washington University, St. Louis, presented the biomarker evidence, concluding that it indicates the treatment modified underlying biology. “These findings support the ability to change the course of Alzheimer’s disease,” he told Alzforum. Takeshi Iwatsubo of the University of Tokyo agreed, saying “This is a monumental event for patients.” All three are co-authors on the NEJM paper. Other researchers mentioned aspects of the trial design that strengthened their confidence in the findings, such as Eisai using separate medical teams to handle participants’ clinical trial evaluations and ARIA to minimize the risk of unblinding.
At the same time, researchers said the ARIA risks need to be taken seriously, and stressed that not all patients will be candidates for this therapy. Everyone agreed on the need to build on a small effect size by adding other therapeutic approaches and finding ways to give lecanemab earlier in disease.
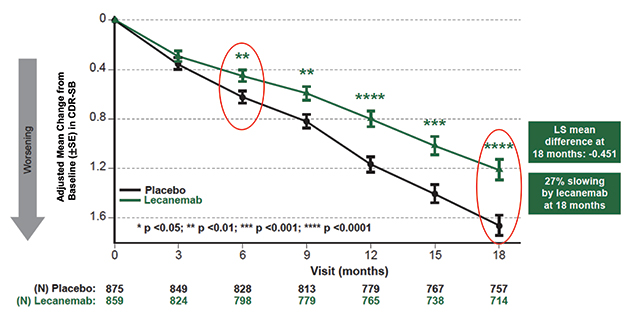
Diverging Trajectories. People on lecanemab worsened more slowly on the CDR-SB than did people on placebo, resulting in a quarter less progression at 18 months. [Courtesy of Eisai.]
A Consistent Clinical Benefit
The 18-month Clarity trial enrolled 1,795 people with mild cognitive impairment or mild dementia due to AD, half of whom received 10 mg/kg intravenous lecanemab every two weeks. Eisai previously reported that lecanemab slowed decline on the primary outcome measure, the CDR-SB, by 0.45 points on the 18-point scale, or about one-quarter of the 1.66-point decline seen in the placebo group (Sep 2022 news).
In San Francisco, Christopher van Dyck of Yale School of Medicine in New Haven, Connecticut, fleshed out details on secondary measures. These mirrored the CDR-SB, with participants on lecanemab declining 1.44 fewer points on the ADAS-Cog14 and 0.05 fewer on the ADCOMS than the placebo group, for relative slowings of 26 and 24 percent, respectively. On a functional measure, the ADCS MCI activities of daily living (ADL), lecanemab put on the breaks by 2 points, or 37 percent. For all four clinical measures, the difference between lecanemab and placebo became statistically significant by six months and grew over time. On the CDR-SB and ADL, the slopes continued to diverge up to 18 months, whereas the difference between the curves appeared to stabilize on the ADAS-Cog14 at 15 months, and on the ADCOMS at 12.
“Because there is such mild decline in these patients over 18 months, it’s very difficult to see a positive signal,” noted Eric Musiek of Washington University in St. Louis, adding, “I’m impressed the signal is so clear, even if it is small in an absolute sense.”
The slightly larger effect on ADLs caught the interest of some scientists, since these can feel most important to participants. “[This] indicates that patients and families could benefit from slowing of observable functional worsening,” Joshua Grill of the University of California, Irvine, wrote to Alzforum (full comments below).
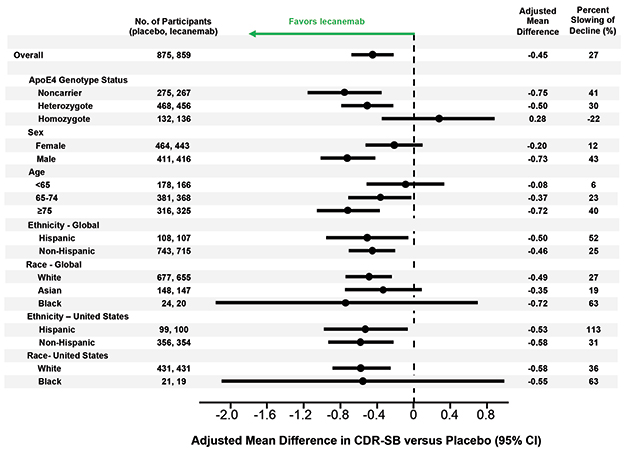
Left of Center. Lecanemab had similar effects in all subgroups examined, though men appeared to benefit more than women, older people more than younger, and APOE4 non-carriers more than carriers. [Courtesy of Eisai.]
Van Dyck also showed results from several sensitivity analyses that suggested the findings were not caused by confounding factors. In the treatment and placebo groups, 81 and 84 percent of participants, respectively, completed the trial, but dropouts did not affect the results. Imputing missing data and accounting for the COVID-19 pandemic did not change the data either. Nor did removing data from participants who developed ARIA-E, suggesting the positive results were not due to inadvertent unblinding of participants.
Likewise, subgroup analyses breaking down participants by age, sex, race, ethnicity, geographic region, disease stage, and use of symptomatic AD medications found treatment benefits across the board. Women appeared to benefit somewhat less than men, a potential difference that sparked discussion in the field. One possibility is that women have more advanced tau pathology at a given stage of cognitive impairment than men, making amyloid removal less effective for them, Maria Teresa Ferretti of the University of Zurich told Alzforum (Nov 2019 news).
The researchers also found a difference by APOE genotype. APOE4 carriers made up two-thirds of the cohort, and seemed to benefit less from lecanemab than noncarriers. In particular, the 15 percent of participants who carried two copies of APOE4 appeared to post no treatment effect on the CDR-SB, and but a small one on the ADAS-Cog14 and ADCS MCI-ADL. However, several scientists told Alzforum that they suspect the homozygote finding represents statistical noise. They pointed out that APOE4 homozygotes on placebo barely declined during the trial, muddying the ability to see a treatment effect in this small subgroup.
Colin Masters of the University of Melbourne, Australia, believes greater effects of lecanemab in APOE4 noncarriers make sense. “We know APOE4 leads to amyloid deposition starting earlier in life than in E4 noncarriers. I suspect the noncarriers had a better result because they started out with a lower amyloid burden,” Masters told Alzforum. He suggested letting trials run longer than 18 months to better detect effects in carriers.
The findings contrast with data from aducanumab, where more of the cognitive benefit in the positive EMERGE trial occurred in APOE4 carriers (see Nov 2020 news).
The Pesky Question: Does This Help Patients?
As with the FDA approval of aducanumab in June 2021, researchers at CTAD debated whether the measured benefit on these clinical tests is clinically meaningful. Sharon Cohen of the Toronto Memory Program, a site investigator for the Clarity trial, argued that it is. She noted that participants on lecanemab and their caregivers reported from one-quarter to one-half less worsening on measures of quality of life and caregiver burden compared to the placebo group. Looking at the data another way, the slower decline translated to a one-third lower risk of advancing to the next stage of AD during the trial, Cohen said.
More Time. Alzheimer’s disease progressed more slowly in people on lecanemab, delaying arrival of the next disease stage. [Courtesy of Eisai.]
This equates to a five- to six-month delay in disease progression, said Eric Siemers of Siemers Integration LLC (full comment below). Others noted this is similar to the benefit of acetylcholinesterase inhibitors, and wanted a Cohen’s d analysis of effect size for easier comparison with other treatments.
Researchers agree that the key question is what happens when people stay on lecanemab for longer periods. Will the clinical benefit persist, grow, as many argue, or diminish? Clinicians are eager to see data from open-label extension studies that might answer this question. “If the reduction in decline were to persist for, say, three to four years, I would expect it to be appreciated by families and patients. On the other hand, if the effect is not durable and fades within a year or so, there will be much less enthusiasm for its use, which after all is somewhat arduous,” David Knopman of the Mayo Clinic in Rochester, Minnesota, wrote to Alzforum (full comment below).
Amyloid as Surrogate?
As expected, lecanemab’s slashing of plaque was dramatic. In Clarity, participants started with an average amyloid PET of 76 centiloids at baseline. This rose by four in the placebo group and dropped by 55 in the treatment group, for a difference of 59 centiloids at 18 months. The divergence between groups became statistically significant at three months, and another three months later clinical measures started changing. Participants on lecanemab ended up with an average of 23 centiloids, which is below the threshold for amyloid positivity typically set at 25. Put another way, two-thirds of the treatment group became PET amyloid-negative at 18 months.
Roger Nitsch of Neurimmune, Switzerland, believes there is also a threshold effect for clinical benefit. In a keynote talk at CTAD, he noted that positive trials of anti-amyloid antibodies, such as Clarity, aducanumab’s EMERGE, and donanemab’s TRAILBLAZER, all brought plaque below 25 centiloids. Negative trials, such as aducanumab’s ENGAGE and the recent GRADUATE studies of gantenerumab, did not. “We have to lower amyloid load to 25 or less to get a clinical effect,” Nitsch proposed.
Others concurred that the relationship between amyloid removal and clinical benefit may not be linear, but stepwise. Eisai has not yet shown data on the correlation between how much of a person’s plaque vanished and their clinical response in Clarity. Ron Petersen of the Rochester Mayo Clinic said that more data are needed on whether it makes a difference how fast amyloid was removed.
At CTAD, researchers debated whether the Clarity results are strong enough to validate plaque removal as a surrogate biomarker for disease slowing. Maria Carrillo of the Alzheimer’s Association made a case for this; others want to see more data. Van Dyck noted that plaques could well be a stand-in for smaller aggregates, which might be the toxic species responsible for cognitive decline. Grill suggested that downstream markers of tangle burden and neurodegeneration may be more important for predicting the cognitive effects of treatment.
Tangle, Inflammation Markers Down. Tau tangle spread slowed on lecanemab (top), while a fluid marker of astrogliosis fell (bottom). [Courtesy of Eisai.]
Alzheimer’s Biomarkers Down, Neurodegeneration Signals Mixed
Regarding those markers, CTAD provided a wealth of data. Bateman reported that on lecanemab, the Aβ42/40 ratio rose by about 60 percent in cerebrospinal fluid and 10 percent in plasma, while p-tau181 dropped around 16-18 percent in both. These measures became statistically different from placebo at six months, in tandem with the clinical benefits. Tau PET showed a slowing but not stoppage of tangle accumulation in the medial temporal lobe, and trends toward slowing in other brain regions, with the PET signal increasing about half as much as in controls. The astrogliosis marker GFAP fell about 15 percent on lecanemab, but rose 10 percent in those on placebo.
“I was particularly pleased to see the significant fall in plasma GFAP levels,” Dennis Selkoe of Brigham and Women’s Hospital, Boston, wrote to Alzforum. “This suggests a notable amelioration of an inflammatory component that is increasingly recognized as a main feature of AD.”
Neurodegeneration markers gave a more ambiguous picture. CSF total tau fell about 4 percent on lecanemab, while rising 15 percent on placebo. Neurogranin, which reflects synapse loss, normalized as well, falling about 15 percent. On the other hand, plasma NfL only trended toward improvement on lecanemab, while CSF NfL did not change.
As has been seen with other amyloid immunotherapies, structural MRI revealed more shrinkage of whole brain and of cortical thickness on lecanemab, and an expansion of the brain’s fluid-filled ventricles. Curiously, however, atrophy in the much smaller hippocampal region slowed. Brain atrophy used to be considered bad, and is used routinely as a diagnostic aid for many brain diseases. Alzheimerologists still do not know what to make of these findings. An early hypothesis—that gray matter shrinkage may reflect amyloid removal—is not in vogue anymore, but nothing else has emerged in its place. Knopman cautioned that the increase in ventricle size in particular deserves further study. “We are willing to ignore this finding now because we have a clinical benefit, which is the gold standard. But we need to keep it in mind,” he said.
Overall, the data support the idea that lecanemab modifies underlying biology, Bateman said. Eric Reiman of Banner Alzheimer’s Institute in Phoenix, whose abiding interest is in enabling prevention, believes these data will help facilitate future prevention trials for a range of drugs by clarifying how biomarker changes predict clinical outcomes.
ARIA Lower, but the Risk Is Real
Safety is a major concern for the widespread use of any amyloid immunotherapy. Eisai and Biogen previously announced that 12.6 percent of people taking lecanemab developed the brain edema known as ARIA-E. In San Francisco, Marwan Sabbagh of the Barrow Neurological Institute in Phoenix showed details. About one-quarter of ARIA-E cases came with symptoms, which were typically mild and cleared up within three to six months. Three people in the trial had severe symptoms, though Sabbagh did not identify what they were.
As expected, a person’s ARIA-E risk was driven by his or her APOE genotype. One-third of APOE4 homozygotes developed ARIA-E, compared to 10 percent of heterozygotes and 5 percent of noncarriers. For reference, in aducanumab’s Phase 3 trials, those percentages were 66, 36, and 20 (Dec 2021 news).
Researchers said the overall risk/benefit calculation favors lecanemab. “I view the safety profile to be acceptable,” Grill said. Nick Fox of University College London agreed. “Any risk is clearly important, but I believe many of my patients would be willing to take such a risk,” he wrote (full comment below).
Nonetheless, they cautioned that clinicians will need to understand well what concurrent illnesses might magnify their patients’ risk so they can counsel them appropriately. Recent reports of two deaths from brain hemorrhage in the lecanemab open-label extension have broadly publicized the issue. One was a man with atrial fibrillation who was taking blood thinners; the other, a woman with cerebral amyloid angiopathy who received tissue plasminogen activator after a stroke (see Science story).
Neither death has been definitively linked to lecanemab, but they have reignited discussion about whether blood thinners and tPA should be contraindicated for people taking lecanemab. Anticoagulant use was allowed in the Clarity trial, in part because Eisai felt it needed to collect these data to learn about the issue, Eisai’s Mike Irizarry told the audience during his presentation. Macrohemorrhages, defined as any brain bleed larger than 1 cm, came in at 0.7 percent in the treatment group, higher than the 0.2 percent in the placebo group. For people on anticoagulants, the rate of macrohemorrhage on lecanemab was 2.4 percent, a more than threefold increase.
“tPA and anti-Aβ antibodies perhaps should not be given to the same AD patient, especially in the presence of CAA,” said Mathias Jucker, Hertie Institute, Tuebingen. Twenty years ago, Jucker’s group described how both tPA and Aβ immunotherapy induced cerebral bleeding in mouse models with CAA, though the scientists did not test the combination (Winkler et al., 2002; Pfeifer et al., 2002).
Stepping Stone to Disease Modification?
With a decision on accelerated approval of lecanemab scheduled by January 6, researchers expect the drug to become clinically available in 2023 (Jul 2022 news). Fox noted that this will create a tremendous challenge for healthcare systems, which at present lack the resources to do the diagnosis, counseling, imaging, IV infusions, and MRI monitoring needed. Most clinics are unprepared to roll out amyloid immunotherapy quickly at a large scale.
Even so, researchers at CTAD liked that the Clarity cohort was more representative of the general AD population than were previous trial cohorts. It included 22.5 percent Hispanic and 4.5 percent black participants. The age range was broad, from 50 to 90 years old, and inclusion criteria were intentionally liberal, allowing people with common conditions such as hypertension, diabetes, obesity, hyperlipidemia, and heart disease to join. Selkoe noted that about one-fourth of AD patients in the general population would meet the Clarity inclusion criteria, suggesting many could qualify for lecanemab treatment.
Still, clinicians are cautious. “If I were to start using this in my clinic, I would target it at healthier patients with positive biomarkers but milder symptoms, less atrophy on MRI, no microhemorrhages, and no anticoagulation,” Musiek wrote. “Patients will need to be motivated, reliable, and have good access and support (as well as insurance) to successfully receive this therapy and keep up with the MRI monitoring.”
The Alzheimer’s Association in 2021 announced a registry study, dubbed ALZ-NET, to track the long-term risks and benefits of disease-modifying AD therapies (Nov 2021 conference news; Aug 2022 conference news). In San Francisco, Carrillo noted that ALZ-NET enrolled its first patient November 1. So far, all participating clinics are on the east coast of the U.S.; the association invites additional institutions to join.
The FDA already has a mechanism to ensure that risky drugs are safely administered. The Risk Evaluation and Mitigation Strategy (REMS) educates physicians on how best to use drugs with potential deleterious effects, and maintains a central database to track outcomes. Jason Karlawish of the University of Pennsylvania, Philadelphia, suggested the FDA require lecanemab use REMS. “The risk/benefit assessment is an ethically challenging Gordian knot. FDA and CMS should collaborate to assure this complicated drug’s transition from research into practice is net beneficial,” he wrote (full comment below).
In anticipation of approval and, potentially, insurance coverage, changes are already being set in motion across the research field. For example, leaders of longstanding observational cohorts that never disclosed amyloid status to their participants, such as AIBL and others, anticipate contacting them to inform them they can now find out their status and go on lecanemab if appropriate. Down’s syndrome researchers are considering running trials in the readiness cohorts they have been building among this population. Scientists at drug companies across the field are starting to prepare for testing their investigational drugs against lecanemab as background medication. And aducanumab researchers are hinting that—just you wait—this antibody is going to come off the sidelines in 2023, as well.
All researchers Alzforum spoke with stressed that lecanemab and other amyloid immunotherapies represent the beginning of disease-modifying therapies. Many estimate a 30 percent slowing may be the most that can be achieved with amyloid removal alone in a symptomatic population, especially in the presence of mixed pathology. They emphasized the need to explore anti-amyloid drugs in presymptomatic populations with biomarker evidence of only amyloid pathology, or amyloid and tau pathology. All eventually want active vaccines to reduce cost and open treatment to large populations across nations. Finally, they urge combination trials of different therapeutic approaches to build on this first signal. At CTAD, Lefkos Middleton of Imperial College London said, “We need to celebrate what we have, but keep investigating AD biology to find treatments that make a big difference.”—Madolyn Bowman Rogers & Gabrielle Strobel
Therapeutics Citations
News Citations
- ApoE4 and Tau in Alzheimer’s: Worse Than We Thought? Especially in Women
- Aducanumab Still Needs to Prove Itself, Researchers Say
- Aduhelm Phase 3 Data: ARIA Is Common, Sometimes Serious
- Lecanemab: FDA Set Accelerated Approval Decision for January 2023
- Aduhelm Lowers Tau; Registry to Track Real-World Performance
- Bringing Aduhelm—and Antibodies to Come—Into Practice
Paper Citations
-
Winkler DT, Biedermann L, Tolnay M, Allegrini PR, Staufenbiel M, Wiessner C, Jucker M.
Thrombolysis induces cerebral hemorrhage in a mouse model of cerebral amyloid angiopathy.
Ann Neurol. 2002 Jun;51(6):790-3.
PubMed. -
Pfeifer M, Boncristiano S, Bondolfi L, Stalder A, Deller T, Staufenbiel M, Mathews PM, Jucker M.
Cerebral hemorrhage after passive anti-Abeta immunotherapy.
Science. 2002 Nov 15;298(5597):1379.
PubMed.













![Best Weight Loss Supplements [2022-23] New Reports!](https://technologytangle.com/wp-content/uploads/2022/12/p1-1170962-1670840878.png)




