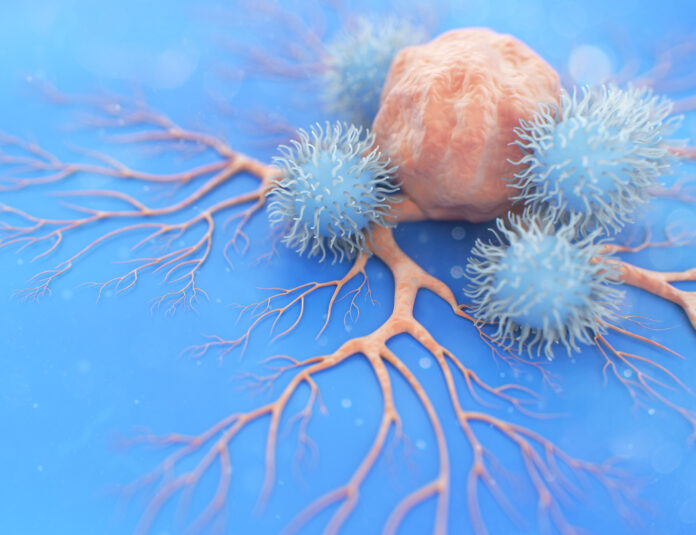
Researchers at the University of Texas MD Anderson Cancer Center have reported encouraging safety and efficacy results from a Phase I trial evaluating a novel cell therapy approach involving natural killer (NK) cells complexed with a bispecific antibody. The study, published in Nature Medicine, showed an overall response rate of 92.9% and a complete response of 66.7% in a cohort of 42 heavily pretreated lymphoma patients.
“These data lend to this approach being considered as a possible curative treatment for some patients and a bridge to a stem cell transplant for others,” said Yago L. Nieto, MD, PhD, professor of stem cell transplantation and cellular therapy at the MD Anderson Cancer Center.
Patients diagnosed with CD30-positive lymphomas can benefit from treatment with Adcetris (brentuximab vedotin), an antibody-drug conjugate targeting CD30, and, in the case of Hodgkin lymphoma, from anti-PD1 checkpoint inhibitor therapy. However, options are limited for those patients whose tumors have become refractory to both treatments, who often face a very poor prognosis.
The experimental treatment makes use of AFM13, a bispecific antibody developed by Affimed that binds to both CD16A on the surface of NK cells and CD30 on lymphoma cells. NK cells derived from cord blood were activated with cytokines, expanded in the presence of antigen-presenting cells and complexed with AFM13 before infusion.
The trial enrolled 42 patients who had received a median of seven lines of therapy prior to the study; all were refractory to both brentuximab vedotin and anti-PD1 immune checkpoint inhibitors. Five participants were diagnosed with T-cell lymphoma and 37 with CD30-positive Hodgkin lymphoma.
The patients received two to four cycles of chemotherapy, followed by an infusion of the allogeneic NK cells complexed with AFM13 at three different dose levels and three weekly intravenous infusions of the bispecific antibody.
The primary endpoint of the trial, establishing the safety of the treatment, was met. No cytokine release syndrome, neurotoxicity, or graft-versus-host disease were reported during the study. In addition, the highest dose of NK cells was selected as the recommended Phase II dose.
Secondary endpoints yielded overall and complete response rates of 92.9% and 66.7%, respectively. Among patients with Hodgkin lymphoma, the overall response rate and complete response were 97.3% and 73%, respectively. The two-year event-free and overall survival rates were 26.2% and 76.2%, respectively, with a median follow-up time of 20 months.
A total of 11 patients remained in complete response for at least 14 months, with some of them maintaining it for up to 40 months after receiving the treatment. Five of them maintained complete remission without any additional therapy, while six went on to receive a stem cell transplant.
“We observed rapid and strong responses to this novel approach of treating patients with AFM13-NK, and we continue to evaluate the efficacy of this therapy for these hard-to-treat malignancies,” said Nieto. “This approach (…) not only holds promise for the treatment of Hodgkin lymphoma but also supports future research into the clinical applications of NK cells with bispecific engagers.”







![Best Weight Loss Supplements [2022-23] New Reports!](https://technologytangle.com/wp-content/uploads/2022/12/p1-1170962-1670840878.png)




