Introduction
The estimated prevalence of CKD was up to 8.2% in China in 2018 to 2019.1 As the renal function of patients with CKD declines progressively, they will eventually develop into end-stage renal disease (ESRD).2 ESRD refers to the inability of the kidneys to maintain fluid, electrolyte, and waste balance in the body and is a major public health challenge worldwide.2 Maintenance hemodialysis is an effective and commonly used renal replacement therapy for patients with ESRD.3 Currently, approximately 89% of dialysis patients worldwide are on hemodialysis.3
Stroke, a major cause of death worldwide, is classified as ischemic or hemorrhagic.4 Stroke is the leading cause of death in China and the estimated prevalence, incidence, and mortality rate of stroke in China in 2020 were up to 2.6%, 505.2 per 100 000 person-years, and 343.4 per 100 000 person-years, respectively.5 Acute ischemic stroke (AIS) is caused by a decrease in blood supply to a certain area of the brain due to vascular obstruction, and has been reported as the most common type of stroke.6 Studies have reported that the prevalence of AIS in hemodialysis patients is 5–10 times higher than that in the general population.7 Previous studies have suggested that a decreased proportion of peripheral CD4 naive T cells, low levels of serum uric acid and serum phosphate levels, and high levels of plasma NT-proBNP may be risk factors for acute ischemic stroke in patients on hemodialysis.8–13 In addition, AIS in hemodialysis patients has a higher mortality rate, higher disability rate, and worse prognosis than in the general population, which seriously affects the quality of life of hemodialysis patients.14 Identifying patients at high risk of AIS as early as possible can help physicians actively prevent and intervene, which has important clinical implications.15
Hyperactive platelets play a key role in thrombosis, leading to acute thrombotic events including AIS.16,17 Circulating platelets vary in their volume and activity.18 Numerous studies have shown that large platelets contain denser granules and produce more thromboxane A2 with higher activity and thrombogenic potential than small platelets.19 The mean platelet volume (MPV) reflects the average size of platelets in a blood sample, and is detected and analyzed automatically by a machine.20 MPV has attracted considerable attention as a potential marker of platelet function and activation, and has been reported to be associated with various thrombotic diseases.17,21 The MPV can predict the occurrence and prognosis of cardiovascular disease.22 It is positively associated with restenosis incidence after coronary angioplasty.23 In addition to MPV, platelet counts also have a predictive value for thrombosis-related diseases. Patients with acute coronary syndrome have been reported to have larger platelet volumes and lower platelet counts than those with stable angina.24 Bessman et al revealed that MPV and platelet counts generally correlate negatively, and total platelet mass tends to be constant in normal subjects.25 Several studies have shown that the mean platelet volume/platelet count ratio (MPR) is also an independent predictor of thrombosis-related diseases such as myocardial infarction, stent thrombosis, and peritoneal dialysis.26–29 However, the predictive value of the MPR for AIS in hemodialysis patients has not yet been reported. Therefore, our study aimed to explore whether MPR has a predictive value for AIS in hemodialysis patients and the relationship between MPR and outcome of AIS.
Materials and Methods
Study Design and Subject Enrollment
The maintenance hemodialysis patients were screened from January 1, 2015, to June 30, 2022, in the Department of Nephrology, Beijing Chao-Yang Hospital. The inclusion criteria were as follows: age > 18 years, kidney failure requiring maintenance hemodialysis, duration of hemodialysis > 3 months, receiving hemodialysis three times a week for 4 hours at the dialysis center, agreed to participate in this experimental study, and voluntarily signed an informed consent form. The exclusion criteria were as follows: coagulation dysfunction caused by severe liver disease and blood system diseases; platelet-related genetic disorders; use of drugs causing thrombocytopenia, such as hydroxyurea, anti-tumor drugs, etc.; thrombocytopenia < 50 * 10 ^ 9/L; patients with an incomplete or unclear medical history; and lack of laboratory data (platelet count, MPV).
A total of 402 patients were screened from January 1, 2015, to June 30, 2022, in the Department of Nephrology, Beijing Chao-Yang Hospital. 143 patients of them were excluded and 259 patients were enrolled in this study. The details of the participant recruitment are presented in Figure 1. This study was conducted in accordance with the Ethics of Clinical Research (Declaration of Helsinki) and approved by the Ethics Committee of Beijing Chao-Yang Hospital (No.2023-Ke-19). All the patients signed a written informed consent form before participating in the study.
 |
Figure 1 Flow diagram of the study population. |
Data Collection
Sex, age, duration of dialysis, primary disease diagnosis, smoking history, diabetes, atrial fibrillation, hypertension, coronary artery disease, previous stroke history, erythropoietin (EPO) dosage at baseline, use of antiplatelet drugs, dialysis anticoagulation modality, and 1-week dialysis mean ultrafiltration fluid loss were obtained from the patients’ medical history and questionnaires. The first occurrence of AIS during follow-up was defined as the outcome. All patients were dialyzed three times a week, and venous blood samples were collected uniformly before the second hemodialysis, that is, in the middle of the week. Blood samples were sent to the same laboratory for testing within 1 h of collection to reduce errors. Routine blood examinations were conducted using an automatic blood cell analyzer (SYSMEX, Japan) and blood biochemical examinations were performed using an automatic biochemical autoanalyzer (Siemens, Germany). All hemodialysis patients were treated with Nikkiso DBB-26 or DBB-27 dialysis machines, F7 polysulfone membrane dialyzers from Feisen, Germany, bicarbonate dialysate from Weigao with a blood flow of 250 to 300 mL/min, and dialysate flow of 500 mL/min. MPR is the ratio of mean platelet volume to platelet count. The patients enrolled in this study were divided into three groups based on the tertiles of the MPR value (Q1, Q2, and Q3).
Outcome Measures
AIS was diagnosed according to the guidelines published by the American Heart Association/American Stroke Association,30 and the outcome was assessed using the modified Rankin Scale (mRS), which was scored at the end of 3 months after the first stroke.31 The mRS score consists of seven scales:0, no symptoms at all; 1. no obvious disability: able to perform daily duties and activities despite symptoms; 2. minor disability: unable to perform all previous activities but able to take care of their own affairs without assistance; 3. moderate disability: requires some assistance but is able to walk without assistance; 4. moderate to severe disability: need assistance to walk and physical requirements; 5) severe disability: bedridden, incontinence, need constant help and attention; 6. death.32 The scores were dichotomized into good (0–2) or poor (3–6) outcome.32
Statistical Analysis
Normally distributed continuous variables are expressed as mean ± standard deviation and compared using an unpaired Student’s t-test. Non-normally distributed variables are expressed as median (interquartile range) and compared using the Mann–Whitney U-test. Categorical variables are expressed as numbers (percentages) and compared using chi-square analysis. Logistic regression analysis was used to explore the risk factors of AIS in patients undergoing hemodialysis. Receiver-operating characteristic (ROC) curve and area under the curve (AUC) were used to evaluate the predictive power of the models. Kaplan-Meier curves were used to investigate the association between the MPR and AIS-free survival in hemodialysis patients. Chi-square analysis was performed to explore the association between the MPR and AIS outcomes in hemodialysis patients. Statistical analyses were performed using the SPSS 19.0 software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism software version 6 (GraphPad Software Inc., San Diego, CA, USA). Statistical significance was set at p < 0.05. Two-tailed p-value was set in our manuscript.
Results
A total of 402 maintenance hemodialysis patients were screened. Three patients excluded due to bone marrow suppression. One patient excluded due to secondary hypersplenism due to cirrhosis. Six patients excluded due to thrombocytopenia. Fifteen patients excluded due to hemodialysis less than 3 months. 49 patients excluded due to follow-up time <5 years and the outcome was unclear. Nine patients excluded due to decline to participate in this study. Five patients excluded due to missing data on relevant variables. 55 patients excluded due to drop out early. Finally, a total of 259 patients were enrolled in this study. A flowchart of the patient selection process is shown in Figure 1.
Comparison of the Medical History and Treatment Between the Two Groups
The present study compared medical history and treatment between the two groups. As shown in Table 1, age (p<0.001), diabetes history (p=0.004), AIS history (p<0.001), CAD history (p=0.024), and dialysis ultrafiltration volume (p<0.033) were greater in the AIS group than in the non-AIS group. However, sex, dialysis duration, systolic blood pressure, diastolic blood pressure, mean arterial pressure, atrial fibrillation history, smoking, aspirin administration, clopidogrel administration, LMH administration, and erythropoietin dosage were not significantly different between the two groups (all p<0.05).
 |
Table 1 The Baseline Characteristics of the Medical History and Treatment in the Two Groups |
Comparison of the Laboratory Test Indicators Between the Two Groups
When the laboratory test indicators were compared between the non-AIS group and AIS group (Table 2), the MPV (p<0.001), MPR (p<0.001), total cholesterol (p=0.009), LDL (p=0.023), and triglyceride (p=0.001) levels were higher in the AIS group than in the non-AIS group. The platelet count (p<0.001), serum albumin (p=0.001), HDL (p=0.045), serum creatinine (p=0.027), and serum uric acid (p=0.021) levels were lower in the AIS group than in the non-AIS group. However, the levels of hemoglobin, serum urea, calcium, phosphorus, fasting blood glucose, iPTH, ferritin, and hsCRP were not significantly different between the two groups (all p<0.05).
 |
Table 2 The Baseline Characteristics of the Laboratory Test Indicators in the Two Groups |
The Prediction Efficiency of MPV for AIS in the Hemodialysis Patients
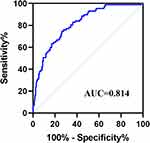
Next, this study further explored the prediction efficiency of MPR for AIS in hemodialysis patients (Figure 2). The ROC curve showed that the AUC is 0.814, with a high sensitivity (0.747) and high specificity (0.738).
Association Between MPR and AIS-Free Survival of Hemodialysis Patients
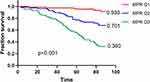
To investigate the association between MPR and AIS-free survival in hemodialysis patients, this study performed a Kaplan–Meier analysis (Figure 3). The patients were divided into three groups according to tertiles (Q) of the MPR value: Q1 (0.021–0.051), Q2 (0.052–0.072), and Q3 (0.073–0.133). The AIS-free survival rates in the three groups were 0.930, 0.701, and 0.360, respectively. Kaplan-Meier analysis indicated that AIS-free survival rates decreased significantly with an increase in the MPR. Log rank tests demonstrated that the differences among the three groups were statistically significant (all p< 0.001).
Association Between of MPR and Outcome of AIS in Hemodialysis Patients
To explore whether MPR and AIS outcomes are associated with patients undergoing hemodialysis. The AIS outcome was evaluated using the mRS score, and the proportion of patients at every mRS scale was calculated. Comparisons were made between hemodialysis patients in the highest quartile (MPR Q3) group and in the combined quartiles MPRQ1-Q2 group (Figure 4). Chi-square analysis revealed that the proportion of patients with a good outcome (mRS 0–2) was significantly greater among patients in the MPR Q1-Q2 group than in the MPR Q3 group (0.844 vs 0.745, p <0.001).
Discussion
In recent years, the incidence of AIS in hemodialysis patients has gradually increased compared with that in patients with hemorrhagic stroke.33 Moreover, AIS in hemodialysis patients is characterized by high mortality, high disability rate, and a high proportion of rehabilitation treatment after stroke.14 In addition to high morbidity, hemodialysis patients with AIS have a worse effect of intravenous thrombolytic therapy than non-dialysis patients.34 Therefore, it is of great clinical significance to identify simple, inexpensive, and effective predictors to identify high-risk AIS in hemodialysis patients and to take preventive measures. MPV in hemodialysis patients is affected by many factors, such as the inflammatory response35 and EPO application,36 and is related to the occurrence of thrombosis-related diseases.17,21 However, there are no relevant reports on the predictive value of MPR for AIS. This study explored the predictive value of the MPR for AIS in hemodialysis patients through a series of analyses.
First, this study explored whether the medical history differed between the two groups. The results demonstrated that hemodialysis patients who were older and had a longer diabetes, AIS, and CAD history had a higher incidence of AIS. This study indicated that older age, history of diabetes, AIS, and CAD were risk factors for ischemic stroke in hemodialysis patients, which is consistent with the trend in the general population.
Next, we investigated whether laboratory test indicators differed between the two groups. Our study revealed that the levels of MPV and MPR were higher in the AIS group than in the non-AIS group, while the level of platelet count is opposite. Platelet volume is negatively correlated with platelet age, with younger platelets showing stronger platelet activity.37 Large young platelets contain more prethrombotic cytokines, such as P-selectin, serotonin, adenosine diphosphate, and β-thromboglobulin, and express more adhesion receptors, such as glycoproteins Ib, IIb, and IIIa, which are involved in vascular endothelial injury, inflammatory responses, and thrombosis.38 The increase in MPV in hemodialysis patients with AIS may be due to platelet activation, decreased consumption, and an increased proportion of young platelets. Studies have shown that decreased platelet counts in cardiovascular diseases may be associated with the activation of the coagulation system and increased glycoprotein VI and inflammatory markers.39,40 This may explain why MPV levels were higher and platelet counts were lower in the AIS group than in the non-AIS group. Several studies have shown that the MPV/PLT ratio has greater diagnostic value for predicting platelet reactivity than MPV alone. Azab et al showed that the MPV/PLT ratio was an independent predictor of 4-year mortality after myocardial infarction, whereas this result was not observed for MPV.26 Han et al also reported that the MPV/PLT ratio had a higher sensitivity and specificity for detecting DVT compared to the use of MPV alone.41 In this study, the ROC curves indicated that MPR had a high accuracy (AUC =0.814) for predicting AIS in hemodialysis patients. The sensitivity and specificity of MPR were 74.713% and 73.837%, respectively. This indicates that the MPR can be used as a good predictor of AIS in hemodialysis patients. Our study also revealed that total cholesterol, LDL, and triglycerides were higher in the AIS group than in the non-AIS group, but HDL levels showed an opposite trend. This study indicated that dyslipidemia is risk factors for ischemic stroke in hemodialysis patients.
In addition, this study also found that the serum albumin, serum creatinine (Scr) and SUA levels were lower in the AIS group than in the non-AIS group. Studies have shown that hypoalbuminemia is not only an independent risk factor for ischemic stroke but also related to its severity and outcome.42 Studies have shown that timely albumin supplementation in ischemic stroke patients with hypoalbuminemia can improve outcomes and reduce mortality.43 Therefore, this study suggests that low albumin level is also a risk factor for AIS in maintenance hemodialysis patients, and timely albumin supplementation can help reduce the risk of AIS and improve prognosis. Some studies have reported inconsistent conclusions regarding the relationship between uric levels and the incidence of AIS. Liu et al found that SUA is a protective factor against stroke severity after AIS in young patients.44 However, Tariq et al reported that an increasing uric acid level was a high-risk factor for stroke.45 In addition, some studies have reported a U-shaped relationship between uric acid levels and adverse outcomes in AIS.46 This study found that hemodialysis patients with higher uric acid levels had a higher incidence of AIS, which may require more studies in the future to clarify the reasons. However, the relationship between SCr levels and AIS incidence remains unclear. SCr is usually used in combination with other indicators to predict the risk and evaluate the prognosis of stroke. Some studies have reported that the SUA/SCr ratio is positively correlated with the risk of ischemic stroke recurrence in young stroke patients;47 the decrease in blood urea nitrogen/SCr ratio was positively correlated with early neurological improvement in AIS patients;48 and the ratio of SCr/ Cystatin C at admission can be used as a predictor of 30-day mortality and long-term poor prognosis in patients with AIS.49 Considering that the 1-week dialysis mean ultrafiltration fluid loss can indirectly reflect the hemodynamic changes in patients during dialysis, the differences in ultrafiltration fluid loss between the two groups were analyzed. The results showed that dialysis ultrafiltration volume was greater in the AIS group than in the non-AIS group. A possible explanation may be that hemodialysis patients have impaired autoregulation owing to reduced baroreflex sensitivity and peripheral arterial compliance caused by atherosclerosis. The brain is susceptible to reduced blood flow, and the mean flow velocity of the cerebral arteries decreases significantly during dialysis; therefore, the hemodynamics of patients may change during dialysis, resulting in a higher risk of AIS.
To investigate the association between MPR and the incidence of AIS in hemodialysis patients, this study performed a Kaplan–Meier analysis, and patients enrolled in this study were divided into three groups based on the tertiles of the MPR value (Q1, Q2, and Q3). The AIS-free survival rates in the three groups were 0.930, 0.701, and 0.360, indicating a higher incidence of AIS in hemodialysis patients with an increase in the MPR value.
Finally, the relationship between baseline MPR and prognosis three months after AIS was also observed in this study. The outcome of AIS was evaluated using mRS scoring, which is simple, time-saving, easily accepted by patients and evaluators, and can accurately assess the level of functional disability in stroke patients. In this study, we calculated the proportion of patients in every scale of the mRS and compared the differences between hemodialysis patients in the highest quartile (MPR Q3) group and in the combined quartiles MPRQ1-Q2 group. The results revealed that the proportion of patients with a good outcome (mRS 0–2) was significantly greater in the MPR Q1-Q2 group than in the MPR Q3 group. This implies that the higher the MPR level, the worse the functional outcome of AIS in hemodialysis patients.
This study has several advantages. First, the MPR was obtained from the ratio of MPV to platelet count, both of which are common indicators in routine blood examinations. It is noninvasive, inexpensive, and convenient. In addition, these two indicators need to be regularly monitored in the medical quality control of hemodialysis patients and have a high detection frequency; the results are also highly accurate and reliable. Second, the experimental design of the study was rigorous. To ensure comparability, the variables might have a potential impact on MPR, such as anticoagulation treatment, and the dosage of erythropoietin were not significantly different between the two groups. Finally, the follow-up time in this study was at least 60 months, and some were as long as 90 months, which helped us study the risk predictors of AIS in hemodialysis patients and obtain reliable results. However, this study had some disadvantages. This was a single-center study, and multicenter and larger cohort studies are expected to further confirm the predictive performance of the MPR for AIS occurrence in hemodialysis patients. In addition, further research is required to determine whether these findings are universal across ethnic groups and countries.
In conclusion, the MPR can be used as a good predictor of AIS in patients undergoing hemodialysis. Patients on hemodialysis with increased MPR levels had a higher incidence of AIS and poorer functional outcomes than those with low MPR levels.
Acknowledgments
This work was supported by grants from the Multi-disciplinary Clinical Research Innovation Project (no. CYDXK202213), the Science and Technology Innovation Fund of Beijing Chaoyang Hospital (No. 21kcjj-7), the Jinzhongzi project of Beijing Chao-Yang Hospital (No.CYJZ202203).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang L, Xu X, Zhang M, et al. Prevalence of Chronic Kidney Disease in China: results From the Sixth China Chronic Disease and Risk Factor Surveillance. JAMA Intern Med. 2023;183(4):298–310. doi:10.1001/jamainternmed.2022.6817
2. Rosselli D, Rueda JD, Diaz CE. Cost-effectiveness of kidney transplantation compared with chronic dialysis in end-stage renal disease. Saudi J Kidney Dis Transpl. 2015;26(4):733–738. doi:10.4103/1319-2442.160175
3. Himmelfarb J, Vanholder R, Mehrotra R, et al. The current and future landscape of dialysis. Nat Rev Nephrol. 2020;16(10):573–585. doi:10.1038/s41581-020-0315-4
4. Campbell BCV, Khatri P. Stroke. Lancet. 2020;396(10244):129–142. doi:10.1016/S0140-6736(20)31179-X
5. Tu WJ, Zhao Z, Yin P, et al. Estimated Burden of Stroke in China in 2020. JAMA Netw Open. 2023;6(3):e231455. doi:10.1001/jamanetworkopen.2023.1455
6. Herpich F, Rincon F. Management of acute ischemic stroke. Crit Care Med. 2020;48(11):1654–1663. doi:10.1097/CCM.0000000000004597
7. Power A, Chan K, Singh SK, et al. Appraising stroke risk in maintenance hemodialysis patients: a large single-center cohort study. Am J Kidney Dis. 2012;59(2):249–257. doi:10.1053/j.ajkd.2011.07.016
8. Kim YK, Shin SJ, Ihm SH, et al. Association between N-terminal pro-brain natriuretic peptide and acute ischemic stroke in patients on chronic hemodialysis. Int Urol Nephrol. 2010;42(2):537–543. doi:10.1007/s11255-009-9689-8
9. Yamada S, Tsuruya K, Taniguchi M, et al. Association Between Serum Phosphate Levels and Stroke Risk in Patients Undergoing Hemodialysis: the Q-Cohort Study. Stroke. 2016;47(9):2189–2196. doi:10.1161/STROKEAHA.116.013195
10. Chen R, Hu J, Xiang F, et al. Decreased percentage of peripheral naive T cells is independently associated with ischemic stroke in patients on hemodialysis. Int Urol Nephrol. 2017;49(11):2051–2060. doi:10.1007/s11255-017-1691-y
11. Chen TS, Chen CH, Chen CA, et al. Low serum phosphate is associated with ischemic stroke in hemodialysis patients. Clin Exp Nephrol. 2018;22(5):1182–1187. doi:10.1007/s10157-018-1578-y
12. Yamaoka M, Yoshida M, Nakashima A, et al. N-terminal pro-brain natriuretic peptide predicts hospitalization for ischemic stroke in Japanese hemodialysis patients. Clin Exp Nephrol. 2022;26(11):1111–1118. doi:10.1007/s10157-022-02254-5
13. Chen Y, Ding X, Teng J, et al. Serum uric acid is inversely related to acute ischemic stroke morbidity in hemodialysis patients. Am J Nephrol. 2011;33(2):97–104. doi:10.1159/000322966
14. Wetmore JB, Phadnis MA, Ellerbeck EF, et al. Relationship between stroke and mortality in dialysis patients. Clin J Am Soc Nephrol. 2015;10(1):80–89. doi:10.2215/CJN.02900314
15. Sato K, Konta Y, Furuta K, et al. Prognostic factors for acute ischemic stroke in patients undergoing hemodialysis. Clin Exp Nephrol. 2022;26(3):286–293. doi:10.1007/s10157-021-02146-0
16. Boos CJ, Lip GY. Platelet activation and cardiovascular outcomes in acute coronary syndromes. J Thromb Haemost. 2006;4(12):2542–2543. doi:10.1111/j.1538-7836.2006.02250.x
17. Arevalo-Lorido JC, Carretero-Gomez J, Villar-Vaca P. Mean platelet volume predicting carotid atherosclerosis in atherothrombotic ischemic stroke. Ir J Med Sci. 2012;181(2):179–183. doi:10.1007/s11845-011-0755-8
18. Corash L, Tan H, Gralnick HR. Heterogeneity of human whole blood platelet subpopulations. I. Relationship between buoyant density, cell volume, and ultrastructure. Blood. 1977;49(1):71–87. doi:10.1182/blood.V49.1.71.71
19. Bath PM, Butterworth RJ. Platelet size: measurement, physiology and vascular disease. Blood Coagul Fibrinolysis. 1996;7(2):157–161. doi:10.1097/00001721-199603000-00011
20. Jackson SR, Carter JM. Platelet volume: laboratory measurement and clinical application. Blood Rev. 1993;7(2):104–113. doi:10.1016/S0268-960X(05)80020-7
21. Balcik OS, Bilen S, Ulusoy EK, et al. Thrombopoietin and mean platelet volume in patients with ischemic stroke. Clin Appl Thromb Hemost. 2013;19(1):92–95. doi:10.1177/1076029611434528
22. Yang A, Pizzulli L, Luderitz B. Mean platelet volume as marker of restenosis after percutaneous transluminal coronary angioplasty in patients with stable and unstable angina pectoris. Thromb Res. 2006;117(4):371–377. doi:10.1016/j.thromres.2005.04.004
23. Norgaz T, Hobikoglu G, Aksu H, et al. The relationship between preprocedural platelet size and subsequent in-stent restenosis. Acta Cardiol. 2004;59(4):391–395. doi:10.2143/AC.59.4.2005204
24. Ranjith LP, Divya R, Mehta VK, et al. Significance of platelet volume indices and platelet count in ischaemic heart disease. J Clin Pathol. 2009;62(9):830–833. doi:10.1136/jcp.2009.066787
25. Bessman JD, Williams LJ, Gilmer PR. Mean platelet volume. The inverse relation of platelet size and count in normal subjects, and an artifact of other particles. Am J Clin Pathol. 1981;76(3):289–293. doi:10.1093/ajcp/76.3.289
26. Azab B, Torbey E, Singh J, et al. Mean platelet volume/platelet count ratio as a predictor of long-term mortality after non-ST-elevation myocardial infarction. Platelets. 2011;22(8):557–566. doi:10.3109/09537104.2011.584086
27. Osken A, Haci R, Dinc Asarcikli L, et al. Mean platelet volume/platelet count ratio as a predictor of stent thrombosis in patients with ST-segment-elevation myocardial infarction. Ir J Med Sci. 2021;190(3):1095–1102. doi:10.1007/s11845-021-02626-y
28. Tuysuz ME, Dedemoglu M. High mean platelet volume to platelet count ratio as a predictor on poor outcomes after CABG. Gen Thorac Cardiovasc Surg. 2020;68(5):459–466. doi:10.1007/s11748-019-01202-7
29. Zhu Y, Peng F, Chen Y, et al. Mean platelet volume/platelet count ratio and mortality in patients on peritoneal dialysis. Clin Nephrol. 2018;90(3):205–211. doi:10.5414/CN109329
30. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: a Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi:10.1161/STR.0000000000000211
31. Huybrechts JF, Caro JJ, Xenakis JJ, et al. The prognostic value of the modified Rankin Scale score for long-term survival after first-ever stroke. Results from the Athens Stroke Registry. Cerebrovasc Dis. 2008;26(4):381–387. doi:10.1159/000151678
32. Mcarthur K, Fan Y, Pei Z, et al. Optimising outcome assessment to improve quality and efficiency of stroke trials. Expert Rev Pharmacoecon Outcomes Res. 2014;14(1):101–111. doi:10.1586/14737167.2014.870479
33. Paul S, Candelario-Jalil E. Emerging neuroprotective strategies for the treatment of ischemic stroke: an overview of clinical and preclinical studies. Exp Neurol. 2021;335:113518. doi:10.1016/j.expneurol.2020.113518
34. Tariq N, Adil MM, Saeed F, et al. Outcomes of thrombolytic treatment for acute ischemic stroke in dialysis-dependent patients in the United States. J Stroke Cerebrovasc Dis. 2013;22(8):e354–9. doi:10.1016/j.jstrokecerebrovasdis.2013.03.016
35. Sari O, Bashir AM, Abdelwahab A. Early Change in Platelet Count and MPV Levels of Patients Who Received Hemodialysis for the First Time: Mogadishu Somalia Experience. Int J Clin Pract. 2022;2022:1503227. doi:10.1155/2022/1503227
36. Asanuma M, Seino K, Mizuno T, et al. Plasma thrombopoietin level and platelet indices in hemodialysis patients receiving recombinant human erythropoietin. Int J Lab Hematol. 2010;32(3):312–319. doi:10.1111/j.1751-553X.2009.01191.x
37. Troussard X, Vol S, Cornet E, et al. Full blood count normal reference values for adults in France. J Clin Pathol. 2014;67(4):341–344. doi:10.1136/jclinpath-2013-201687
38. Chu SG, Becker RC, Berger PB, et al. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8(1):148–156. doi:10.1111/j.1538-7836.2009.03584.x
39. Bigalke B, Stellos K, Stakos D, et al. Influence of platelet count on the expression of platelet collagen receptor glycoprotein VI (GPVI) in patients with acute coronary syndrome. Thromb Haemost. 2009;101(5):911–915. doi:10.1160/TH08-06-0399
40. Jaremo P, Hansson G, Nilsson O. Elevated inflammatory parameters are associated with lower platelet density in acute myocardial infarctions with ST-elevation. Thromb Res. 2000;100(6):471–478. doi:10.1016/S0049-3848(00)00366-2
41. Han JS, Park TS, Cho SY, et al. Increased mean platelet volume and mean platelet volume/platelet count ratio in Korean patients with deep vein thrombosis. Platelets. 2013;24(8):590–593.
42. Shaikh F, Shaikh FH, Chandio SA. Frequency of Hypoalbuminemia and In-Hospital Mortality in Acute Ischemic Stroke Patients Presenting at a Tertiary Care Hospital, Hyderabad. Cureus. 2021;13(4):e14256. doi:10.7759/cureus.14256
43. Dziedzic T, Pera J, Slowik A, et al. Hypoalbuminemia in acute ischemic stroke patients: frequency and correlates. Eur J Clin Nutr. 2007;61(11):1318–1322. doi:10.1038/sj.ejcn.1602643
44. Liu Y, Liu X, Jia J, et al. Uric Acid and Clinical Outcomes in Young Patients with Ischemic Stroke. Neuropsychiatr Dis Treat. 2022;18:2219–2228. doi:10.2147/NDT.S373493
45. Tariq MA, Shamim SA, Rana SF, et al. Serum Uric Acid – Risk Factor for Acute Ischemic Stroke and Poor Outcomes. Cureus. 2019;11(10):e6007. doi:10.7759/cureus.6007
46. Seet RC, Kasiman K, Gruber J, et al. Is uric acid protective or deleterious in acute ischemic stroke? A prospective cohort study. Atherosclerosis. 2010;209(1):215–219. doi:10.1016/j.atherosclerosis.2009.08.012
47. Sun X, Lv J, Wu Z, et al. Serum Uric Acid to Serum Creatinine Ratio and Risk of Stroke Recurrence in Young Adults with Ischemic Stroke. Neuropsychiatr Dis Treat. 2022;18:2031–2039. doi:10.2147/NDT.S378576
48. Jiang WF, Deng ML. Prognostic impact of blood urea nitrogen/creatinine ratio changes in patients with acute ischemic stroke. Clin Neurol Neurosurg. 2022;215:107204. doi:10.1016/j.clineuro.2022.107204
49. Liu W, Zhu X, Tan X, et al. Predictive Value of Serum Creatinine/Cystatin C in Acute Ischemic Stroke Patients under Nutritional Intervention. J Nutr Health Aging. 2021;25(3):335–339. doi:10.1007/s12603-020-1495-0