Department of Dermatology, Affiliated Hospital of North Sichuan Medical College, Nanchong, Sichuan Province, People’s Republic of China
Correspondence: Xiaojie Ding, Department of Dermatology, Affiliated Hospital of North Sichuan Medical College, 1st Maoyuan South Road, Shunqing District, Nanchong, Sichuan Province, 637000, People’s Republic of China, Tel +8615881788808, Fax +8681715881788808, Email [email protected]
Abstract: Psoriasis and atopic dermatitis are relatively common in clinical practice, but it is rare for the two diseases to co-occur in the same patient. Dupilumab, an anti-IL-4/IL-13 monoclonal antibody, has been approved for treating moderate to severe AD. In addition to its therapeutic effects, dupilumab may induce new cutaneous adverse reactions. We report a rare case of pustular psoriasis induced during the treatment of atopic dermatitis with dupilumab. This case demonstrates the need for caution while treating patients with moderate to severe atopic dermatitis and staying vigilant for the emergence of new symptoms, especially with biological agents.
Case Report
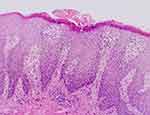
A 13-year-old male child presented to our department in January 2023 with recurrent erythema, papules, vesicles, and oozing accompanied by generalized pruritus for three years. The patient had erythema and papules on the trunk and extremities with no obvious cause three years ago, and after scratching, he developed vesicles, oozing, and crusting. He was diagnosed with atopic dermatitis after several visits to our hospital and other hospitals. The patient’s dermatitis failed to improve after receiving oral anti-allergic and topical steroids: the lesions and itching worsened and seriously affected his quality of life and study, so he revisited our department. He was diagnosed with severe atopic dermatitis upon evaluation and was treated with dupilumab 400 mg subcutaneously, but the lesions worsened, with the appearance of white corn-shaped pustules scattered on his trunk and extremities. We learned through consultation that the patient had a six-year history of allergic rhinitis; his father also had a history of allergic rhinitis, but his mother was healthy. On dermatological examination, diffuse erythema, papules, scales, and mossy plaques were visible on the trunk and extremities, with localized vesicles and exudation. We observed generalized dry skin and a few scattered corn-sized white pustules on the trunk and lower extremities. The patient’s severity of atopic dermatitis score was 80.2 (mild: 0–24; moderate: 25–50; and severe >50) (Figures 1 and 2). Laboratory tests revealed leukocytosis (33,040/μL) with neutrophilia (97,000/μL) and elevated erythrocyte sedimentation rate to improve. Notably, immunoglobulin E (6010IU/mL) and eosinophils (1676/μL, 59.1%) were also elevated. The suggested allergens were house dust mite (>100IU/mL), egg (1.26IU/mL), wheat (0.42IU/mL), and house dust (1.02IU/mL). Based on the appearance of corn-sized white pustules on the trunk and lower extremities of the child, we suspected a new skin disease. Consequently, we performed a dermatopathological biopsy of the white pustules on the child’s abdomen. The biopsy results suggested epidermal hyperkeratosis with fused dyskeratosis, neutrophilic microabscesses in the dyskeratotic stratum corneum, reduced or even absent granular layer beneath the dyskeratosis, hypertrophy of the spinous layer, upwardly displaced dermal papillae, dilated capillaries, superficial perivascular lymphocytes, and scattered neutrophil infiltration (Figure 3).
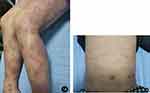 |
Figure 1 (A and B) Typical AD lesions on both lower extremities and trunk. |
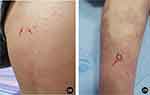 |
Figure 2 (A and B) White pustules on the lower extremities and trunk (red arrow in (A and B)). |
Finally, the child was diagnosed with pustular psoriasis based on his prolonged history of illness for more than six months, clinical presentation, family history, laboratory tests, and histopathological biopsy results. Consequently, we discontinued dupilumab, and the child was continued on anti-allergic and topical moisturizing emollient treatment. The erythematous papules and pruritus reduced considerably after treatment compared to before, and no new pustules appeared after modifying the patient’s treatment. The patient is under regular follow-up.
Discussion
AD and psoriasis are common chronic recurrent inflammatory diseases in dermatology, characterized by T-cell-mediated immune responses and abnormal proliferation and differentiation of keratin-forming cells.1 However, the two diseases differ in that they usually exhibit different T-cell polarity and different cytokine arrays. Therefore, it is rare for psoriasis and AD to coexist in the same patient.2 AD is mainly mediated by T helper (Th)-2, and the cytokines interleukin (IL)-4 and IL-13 are key drivers of its signaling. In contrast, psoriasis is primarily a Th17/Th1 cell-driven disease, and the cytokines Tumor necrosis factor-α (TNF-α), IL-17, IL-22, and IL-23 play an important role in the development of psoriasis.3–6
Dupilumab is a fully human IL-4 receptor subunit monoclonal antibody that targets the IL-4Rα subunit of the IL-4 and IL-13 receptors to block IL-4 and IL-13-mediated downstream signaling and inhibit Th2-mediated immune response, thereby blocking the pathogenesis of AD.7 Dupilumab has been approved by the Food and Drug Administration (FDA) in the United States for treating moderate to severe atopic dermatitis in uncontrolled patients.8 Numerous clinical studies and real-world data have demonstrated the efficacy and safety of dupilumab in treating moderate to severe AD.9,10 Dupilumab can rapidly control skin itches and significantly improve anxiety, depression, and insomnia in AD patients. With the increase in the number of patients using this drug, there have been reports of rare adverse drug reactions, such as facial and neck dermatitis,4 urticaria,5 and lichen ruber planus6 following the use of dupilumab in treating AD. It has been suggested that these skin manifestations are caused by the inhibition of the Th2 pathway by dupilumab.11
In recent years, there have been increasing reports of dupilumab-inducing psoriasis. An FDA study suggested that using dupilumab for AD may cause psoriasis.12 The largest cohort study so far showed that the incidence of new psoriasis in patients receiving dupilumab for the treatment of AD is 1.7%, which is similar to the incidence rate of psoriasis in the general population. The average latency from the start of the drug to the occurrence of psoriasis is 3.7 months.13 Interestingly, our patient had only ten days from the start of dupilumab to the manifestation of pustules, causing us to misdiagnose new-onset psoriasis for worsening atopic dermatitis initially. The clinical manifestations of psoriasis or psoriasis-like lesions after the use of dupilumab in the treatment of AD are diverse, with reported cases involving the common type,14 erythrodermic type,15 and pustular type.16 However, these occurrences are relatively rare and have not been included as adverse events in clinical trials.
Currently, the theory of immune drift is emerging as a hot topic in the mechanism of conversion of AD to psoriasis by biological agents. In healthy individuals, there is a dynamic balance of Th1/Th2. A Th1/Th2 imbalance—when the function of one subgroup is enhanced or that of another subgroup is inhibited—will lead to a reverse transformation of the disease and the occurrence of “immune drift”. This balance may be altered by the suppression or injection of cytokines with immunomodulatory functions. It has been shown that IL-4 and IL-4 receptors contribute to dendritic cell maturation. Dendritic cells enhance Th2 responses, negatively regulate Th1/Th17, inhibit dendritic cell-mediated IL-23 production, and suppress IL-1β and IL-6 secretion by psoriasis epidermal cells. Therefore, blocking IL-4 may induce overexpression of Th1 cytokines, such as IL-23 and IL-17, leading to psoriasis. Mirza et al, found the expression of new IL-17A in psoriasis induced by dupilumab, suggesting that IL-17A produced by T-cell reactive polarization may be a driving factor for dupilumab-induced psoriasis drift in AD patients.12 Their finding further indicates that inhibiting Th2 cytokines may induce a drift toward a Th17/Th1 dominated immune response, leading to psoriasis. Additionally, the induction of AD lesions with the use of Th1 inhibitors provides additional support for the theory of immune drift.16 Fowler et al, suggested that AD is primarily a Th2 disease in the acute phase but shifts partially to Th1 over time as it progresses to the chronic phase. The Th1 response becomes more pronounced with Th2 inhibition by dupilumab. Additionally, psoriasis improves after discontinuing dupilumab, suggesting a causal relationship between dupilumab and the development of psoriasis.17 Furthermore, recessive mutations in IL-36RN may be an important factor in the development of pustular psoriasis after dupilumab injection in AD patients.18 Dupilumab modulates the downstream IL-36 signaling pathway, induces inflammatory keratinocyte responses, and stimulates IL-12 and IL-23 secretion by skin dendritic cells, promoting the differentiation of naive CD4 T cells to Th1 and Th17 cells. Consequently, the process causes the secretion of TNF-α and IL-17A, contributing to the development and progression of the disease.19
Conclusion
New skin lesions that develop during treatment with dupilumab should be carefully identified and monitored. Herein, we report a rare case of pustular psoriasis induced during dupilumab injection for AD. New skin lesions are often overlooked in clinical practice because moderate to severe AD lesions are widespread throughout the body, requiring dermatologists to be more vigilant. Dupilumab has a relatively short marketing time and limited experience in medication. So far, there is no clear explanation to elucidate the relationship between Th2 inhibition by dupilumab and its impact on the pathogenesis of psoriasis.
Abbreviations
AD, Atopic dermatitis; Th, T helper; TNF-α, Tumor necrosis factor –α; FDA, food and drug administration; IL, interleukin.
Ethics and Consent Statement
Informed consent was obtained from the patients’ mother. The written informed consent was signed by the patients’ mother to have the case details and any accompanying images published. Institutional approval was not required to publish the case details.
Acknowledgments
Li Liu and Jie Chen are co-first authors for this study. We would like to thank the patient and physicians for participating in our research.
Funding
The authors declare that this study has received no financial support.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum. Curr Opin Immunol. 2017;48:68–73. doi:10.1016/j.coi.2017.08.008
2. Paolino G, Di Nicola MR, Brianti P, Bianchi VG, Mercuri SR. New onset atopic dermatitis and psoriasis in the same patients under biologic treatments: the role of systemic treatments as a possible trigger. Dermatol Ther. 2022;35(11):e15814. doi:10.1111/dth.15814
3. Barry K, Zancanaro P, Casseres R, Abdat R, Dumont N, Rosmarin D. Concomitant atopic dermatitis and psoriasis-a retrospective review. J Dermatolog Treat. 2021;32(7):716–720. doi:10.1080/09546634.2019.1702147
4. Jo CE, Finstad A, Georgakopoulos JR, Piguet V, Yeung J, Drucker AM. Facial and neck erythema associated with dupilumab treatment: a systematic review. J Am Acad Dermatol. 2021;84(5):1339–1347. doi:10.1016/j.jaad.2021.01.012
5. Mastorino L, Ortoncelli M, Virginia B, et al. Dupilumab-induced urticaria. Dermatol Ther. 2021;34(6):e15117. doi:10.1111/dth.15117
6. Mastorino L, Ortoncelli M, Giura MT, et al. Lichen ruber planus arising during dupilumab treatment for atopic dermatitis. Ital J Dermatol Venerol. 2022;157(5):449–450. doi:10.23736/S2784-8671.21.07070-5
7. Harb H, Chatila TA. Mechanisms of dupilumab. Clin Exp Allergy. 2020;50(1):5–14. doi:10.1111/cea.13491
8. Thibodeaux Q, Smith MP, Ly K, Beck K, Liao W, Bhutani T. A review of dupilumab in the treatment of atopic diseases. Hum Vaccin Immunother. 2019;15(9):2129–2139. doi:10.1080/21645515.2019.1582403
9. Mastorino L, Viola R, Panzone M, et al. Dupilumab induces a rapid decrease of pruritus in adolescents: a pilot real-life study. Dermatol Ther. 2021;34(6):e15115. doi:10.1111/dth.15115
10. Mastorino L, Rosset F, Gelato F, et al. Chronic pruritus in atopic patients treated with dupilumab: real life response and related parameters in 354 patients. Pharmaceuticals. 2022;15(7):883. doi:10.3390/ph15070883
11. Guimarães PM, Scavuzzi BM, Stadtlober NP, et al. Cytokines in systemic lupus erythematosus: far beyond Th1/Th2 dualism lupus: cytokine profiles. Immunol Cell Biol. 2017;95(9):824–831. doi:10.1038/icb.2017.53
12. Mirza FN, Wang A, Ramachandran SM, Damsky W, Cohen JM. Dupilumab-induced phenotype switch from atopic dermatitis to psoriasis is characterized by de novo interleukin-17A expression: a case report. Br J Dermatol. 2021;185(2):432–434. doi:10.1111/bjd.20064
13. Brumfiel CM, Patel MH, Zirwas MJ. Development of psoriasis during treatment with dupilumab: a systematic review. J Am Acad Dermatol. 2022;86(3):708–709. doi:10.1016/j.jaad.2021.05.013
14. Safa G, Paumier V. Psoriasis induced by dupilumab therapy. Clin Exp Dermatol. 2019;44(3):e49–e50. doi:10.1111/ced.13901
15. Tracey EH, Elston C, Feasel P, Piliang M, Michael M, Vij A. Erythrodermic presentation of psoriasis in a patient treated with dupilumab. JAAD Case Rep. 2018;4(7):708–710. doi:10.1016/j.jdcr.2018.05.014
16. Zhong X, Li Y, Gao Y, et al. Pustular psoriasis appearing in a Chinese woman treated with dupilumab for atopic dermatitis: a case report. Dermatol Ther. 2022;35(11):e15851. doi:10.1111/dth.15851
17. Fowler E, Silverberg JI, Fox JD, Yosipovitch G. Psoriasiform dermatitis after initiation of treatment with dupilumab for atopic dermatitis. Dermatitis. 2019;30(3):234–236. doi:10.1097/DER.0000000000000481
18. Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365(7):620–628. doi:10.1056/NEJMoa1013068
19. Cohen JN, Bowman S, Laszik ZG, North JP. Clinicopathologic overlap of psoriasis, eczema, and psoriasiform dermatoses: a retrospective study of T helper type 2 and 17 subsets, interleukin 36, and β-defensin 2 in spongiotic psoriasiform dermatitis, sebopsoriasis, and tumor necrosis factor α inhibitor-associated dermatitis. J Am Acad Dermatol. 2020;82(2):430–439. doi:10.1016/j.jaad.2019.08.023