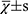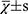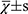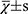Background
With the development of medical technology, cardiac valve replacement surgery skills have been increasingly improved, and the proportion of minimally invasive and off-pump heart surgery has increased.1 While valvular heart disease can be resolved by surgery, the occurrence of postoperative acute kidney injury (AKI) cannot be ignored. Studies have shown that the incidence of AKI after cardiac surgery is 8.9%–40.2%.2–5 Many factors affect the development of AKI after cardiac surgery, including exposure to exogenous (drug) and endogenous toxins, ischemia/reperfusion injury, hemodynamic changes of renal vascular embolization, neuroendocrine activation, metabolic factors, inflammation and oxidative stress. Before cardiac surgery, patients often use potential nephrotoxic drugs, such as antibiotics, non-steroidal anti-inflammatory drugs (aspirin), angiotensin-converting enzyme inhibitors, angiotensin receptor antagonists, and intravenous contrast agents. Among them, NSAIDs damage renal blood flow self-operative use of intravenous contrast agents increases the risk of perioperative AKI. Before surgery, many patients undergoing cardiac surgery will experience one or more hypotension, which causes endothelial damage, endothelin, angiotensin and catecholamine local release, causing renal vasoconstriction, aggravate ischemia and blood pump used in CPB cause mechanical damage to the blood, contribute to the production of oxidative stress and systemic inflammatory response.6
However, in general, clinical judgment of AKI is only based on urine volume, serum creatinine (SCr), and blood urea nitrogen (BUN) levels, which are not sufficiently sensitive for the early diagnosis of AKI and lack specificity. In recent years, increasing attention has been paid to sensitive indicators for early diagnosis of AKI, which can be used to evaluate whether the renal function is damaged and to intervene in the protection of renal function. Several studies have reported on the role of kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL), and heme oxygenase-1 (HO-1) in the early diagnosis of AKI after cardiac surgery.7,8 KIM-1 is a protein greatly expressed in the tubular epithelial cells during renal dysfunction, and in the kidney self-repair sustained high expression and released into the urine and blood, released more in the urine, directly promote the removal and regeneration of the tubular necrotic cells, under normal circumstances it is important that the kidney tissue does not express KIM-1. NGAL is a sensitive tissue marker of renal tubular injury. It was initially found in activated neutrophils, and NGAL is expressed at low levels in human organs, such as kidney, lung, stomach, and colon. It is one of the most prominent genes with elevated expression levels in kidney injury, appearing very early in AKI caused by ischemic injury or nephrotoxicity.
In patients with AKI after cardiac surgery, urinary NGAL levels began to increase at 2 h, at 6 h reaching a peak after CPB. NGAL is a glycoprotein released by damaged renal tubular cells, a sensitive product of clinical and subclinical AKI, and NGAL is stored in the granules of mature neutrophils. It has early diagnosis of AKI in many different clinical settings.
In this study, we analyzed the perioperative expression levels of KIM-1, NGAL, and HO-1 in patients with AKI after cardiopulmonary bypass (CPB) cardiac valve replacement. We explored their early diagnostic value for AKI after cardiac valve replacement.
Materials and Methods
Research Materials and Subgroups
In this study, we enrolled 80 patients diagnosed with rheumatic valvular heart disease in the Department of Cardiovascular Surgery of our hospital from January 2022 to December 2022.
Inclusion criteria: (1) Diagnosis of rheumatic heart disease, with indications for cardiac valve replacement surgery. (2) Signed consent form for cardiac valve replacement surgery. (3) Based on the pre-operation cardiac color Doppler ultrasound, the patients underwent either aortic valve replacement, mitral valve replacement, or double valve replacement.
Exclusion criteria: (1) Age greater than 70 years old. (2) Preoperative diagnosis of infective endocarditis. (3) Secondary valve replacement surgery. (4) Acute and chronic renal insufficiency or existence of combined chronic renal disease before the operation. (5) Preoperative administration of drugs that affect renal function. (6) Secondary transfer and thoracotomy during the operation. (7) Death occurring during or within 48 hours after the operation.
The patients were divided into the AKI group and non-AKI group based on the occurrence of postoperative AKI. The expression levels of urinary KIM-1, NGAL, HO-1, SCr, and BUN in patients between the two groups before the operation and at 12 h, 24 h, and 48 h after the operation, were compared, and the early diagnostic value for postoperative AKI discussed. This study was approved by the Hospital Ethics Committee, all enrolled patients signed an informed consent form, and the type of valve replacement surgery (biological valve/mechanical valve) was selected by the patients and their families.
Diagnostic Criteria of AKI:
“The Guide to Clinical Practice of KDIGO Acute Kidney Injury, 2012” was adopted for the diagnosis and staging of AKI,9,10 which was defined as: an increase in SCr level within 48 h ≥ 26.5 umol/L (0.3 mg/mL), or SCr level exceeds 1.5 times or more of the baseline value, and the above conditions are explicit or inferred to occur within 7 days, or urine volume < 0.5 mL/(kg h) for 6 h. AKI is diagnosed in patients with any one of the above conditions.
Collection of General Clinical Data and Test Data
(1) The age, gender, body weight, past medical history, cardiac valve surgical methods, and perioperative (pre-operation, 12 h, 24 h, and 48 h post-operation) urinary KIM-1, NGAL, HO-1, SCr, and BUN levels of selected patients were collected. At each point during the perioperative period, 5 mL venous blood and 10 mL urine were collected, centrifuged, and stored in a refrigerator at −80°C. (2) Experimental methods: All samples were tested in the central laboratory of our hospital. NGAL and KIM-1 in urine samples were tested by enzyme-linked immunosorbent assay (ELISA), and HO-1, SCr, and BUN were determined by an automatic biochemistry analyzer.
Statistical Methods
The statistical analysis was performed by another blinded statistician using SPSS 19.0 software. The measurement data were expressed as mean ± standard deviation (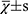
Results
Comparison of General Clinical Data Between the Two Groups
Among the included patients, 22 patients (AKI group) experienced postoperative AKI (met the diagnostic criteria for AKI), the incidence of postoperative AKI was 27.5%, and the remaining 58 patients (non-AKI group) did not experience AKI. There was no significant difference in age, gender, weight, past medical history, or surgical method between the two groups (P > 0.05) (Table 1).
 |
Table 1 Comparison of General Clinical Data of Patients Between the Two Groups ( |
Comparison of KIM-1, NGAL, and HO-1 Levels Between the Two Groups During the Perioperative Period
Compared between AKI group and preoperative group, KIM-1, NGAL, HO-1, blood creatinine, and BUN levels were significantly increased, with significant differences (P <0.05). Compared with non-AKI groups, KIM-1, NGAL, HO-1, blood creatinine, and blood urea nitrogen levels increased at all time points, but the difference was not significant (P > 0.05). Compared with AKI group and non-AKI group, KIM-1, NGAL, HO-1, blood creatinine, and BUN levels increased significantly, the differences were statistically significant (P < 0.05). After the operation, the levels of KIM-1, NGAL, HO-1, SCr, and BUN in the AKI group were higher than those in the non-AKI group. The differences were statistically significant (P < 0.05). The increase in the AKI group was more significant (Tables 2–7).
 |
Table 2 Comparison of KIM-1, NGAL, and HO-1 at Different Point in Times of the AKI Group ( |
 |
Table 3 Comparison of KIM-1, NGAL, and HO-1 at Different Point in Times of the Non-AKI Group ( |
 |
Table 4 Comparison of KIM-1, NGAL, and HO-1 Between the Two Groups ( |
 |
Table 5 Comparison of SCr and BUN at Different Point in Times of the AKI Group ( |
 |
Table 6 Comparison of SCr and BUN at Different Point in Times of the Non-AKI Group ( |
 |
Table 7 Comparison of SCr and BUN in the Two Groups During the Perioperative Period ( |
Discussion
Acute kidney injury after cardiac surgery is one of the main causes of postoperative renal dysfunction. Based on the underlying baseline characteristics and type of surgery, the morbidity is up to 39%, and 1% to 6.5% of patients require renal replacement therapy (RRT). AKI was independently associated with a higher mortality rate (up to 60% in severe cases) and an almost 8-fold increase in the risk of developing chronic kidney disease (CKD) [?]. There is clear evidence in numerous cohort studies that the risk of death is proportional to the severity of kidney injury. With advances in cardiopulmonary bypass (CPB) technology and ICU monitoring, mortality decreases despite the increased incidence of AKI. The pathogenesis of AKI after cardiac surgery is complex and considered to be the result of nephrotoxic substances, ischemia and ischemia reperfusion injury, cardiopulmonary bypass exposure and activation of inflammatory pathways and atheroembolic mechanisms.11
CPB is an essential adjunct technology to complete open heart surgery. The CPB can cause non-physiological blood perfusion and then cause organ function damage by activating inflammatory response and oxidative stress, which is an essential factor affecting the prognosis of patients. AKI after CPB has become a critical complication in clinical work due to its high incidence, complex etiology, poor prognosis, and high mortality. If not treated in time, it may develop into acute renal failure and increase the mortality of patients. The incidence of AKI after heart valve replacement in our study was 27.5%, which is consistent with the reports in domestic studies.2–5 When AKI occurs, it becomes an independent risk factor for prolonged hospitalization and increased mortality. Therefore, it is crucial to explore early sensitive indicators of postoperative AKI and to start renal protection measures as soon as possible.
KIM-1 is a newly discovered type I transmembrane protein that belongs to the family of T lymphocytes, with a relative molecular mass of 104,000 and almost no expression in normal human and animal kidneys. However, in ischemia and nephrotoxic injury, the protein intracellular signal peptide can be cleaved by matrix metalloproteinase (MMP) in the early stage of injury, released into the renal tubules, and excreted in the urine.
Gelase-associated apolipoproteins are considered members of the NGAL family and a protein molecule that regulates apoptosis of renal tubular epithelial cells in renal tissues. They are rarely found in renal tissues, and only genes are upregulated in renal tissues after stress or after trauma like renal ischemia. And further studies found that the level of urine and blood NGAL values increased significantly during renal ischemia-reperfusion injury. However, renal injury in renal ischemia was clearly upregulated in renal tissue and could be detected in urine within hours.
Recent studies have suggested12,13 that KIM-1 and NGAL increase significantly after renal ischemia. A recent study on KIM-114 has confirmed the potential role of KIM-1 as a new biomarker for early diagnosis of AKI. Patients with valvular heart disease undergoing cardiac surgery with CPB have an increased risk of AKI and may develop the cardiorenal syndrome. In a group of 113 adult patients undergoing CPB,13 the incidence of AKI (Criterion: SCr increase greater than 0.3 mg/dl) was 31% and urinary KIM-1 level of patients with AKI in this group increased by 40% at 2 hours after surgery and more than doubled at 24 hours after surgery. It is evident that KIM-1 can reflect the condition of AKI earlier than conventional detection methods. In this study, the urinary KIM-1 and NGAL levels at 12 h, 24 h, and 48 h after surgery in the AKI group were significantly higher than those before surgery and the increases were significant when compared with those in the non-AKI group, which is consistent with the previous study reports.
Gelase-associated apolipoproteins are considered members of the NGAL family and a protein molecule that regulates apoptosis of renal tubular epithelial cells in renal tissues. They are rarely found in renal tissues, and only the genes are upregulated in NGAL after stress or after trauma like renal ischemia. And further study found that the level of urine and blood NGAL value increased significantly during renal ischemia and reperfusion injury. However, renal injury in renal ischemia was clearly upregulated in renal tissue and could be detected in urine within hours. Under normal physiological conditions, NGAL can also be expressed in many human tissues, such as bronchi, stomach, colon, pancreas, kidney, and other tissues. NGAL expression is usually low and significantly increases in case of an inflammatory injury. As a result, NGAL expression is caused by the interaction between inflammatory cells and epithelial cells. Activation of inflammatory reaction and damage of renal tubular epithelial cells during CPB surgery can stimulate renal epithelial cells to secrete a large amount of NGAL. Therefore, the concentration of NGAL in the urine can reflect the degree of renal injury. NGAL can predict the occurrence of AKI after CPB and can be used to evaluate the prognosis. Bennett et al15 found that urinary NGAL levels after CPB operation increased 15 times at 2 h and up to 25 times at 4–6 h, and the urinary NGAL level at 2 h was correlated with the severity and prognosis of AKI. A systematic retrospective analysis found that NGAL was more valuable for the diagnosis of early AKI and also for the prognostic judgment of AKI, with the higher the NGAL level, the greater the severity of AKI.7 Kidher et al16 found that the postoperative NGAL level can be used as an early and influential biomarker to predict the occurrence of postoperative AKI and the need for intervention. In this study, we found that the postoperative NGAL level in the AKI and non-AKI groups was higher than before surgery, and the difference was statistically significant. However, the NGAL level in patients in the AKI group was elevated more significantly.
The early diagnostic effect of heme oxygenase-1 (heme oxygenase-1, HO-1) in cardiac AKI after cardiopulmonary bypass has been reported in the literature. HO-1 is a widespread antioxidant defense enzymes in living organisms, and various inducing factors, such as heme, hyperoxia, oxygen, hypoxia, heat shock, endotoxin, and cytokines can significantly increase the expression of HO-1. Another clinical study17 reported that compared with patients without AKI, chronic kidney disease and end-stage renal disease in ICU, patients with AKI in ICU had significantly increased HO-1 in serum and urine. With respect to the research on HO-1 and cardiac surgery, Billings et al18 showed that for patients with AKI after cardiac surgery, the HO-1 level was significantly increased, and the increased level of HO-1 was related to the CPB duration, intraoperative hemolysis, and inflammatory reaction. In this study, we found that after cardiac valve replacement, HO-1 levels of patients in the AKI and non-AKI groups were higher than before the operation. However, the increase in the AKI group was significant and the difference was statistically significant (P<0.05).
Conclusion
To sum up, the levels of KIM-1, NGAL, and HO-1 in patients after CPB cardiac valve replacement are increased, but the increase in patients with AKI is more significant. The combined monitoring of the three can provide early warning for AKI after surgery, and is conducive to guiding the early start of renal function protective measures and improving the clinical prognosis.
Abbreviations
AKI, acute kidney injury; KIM-1, kidney injury molecules-1; NGAL, neutrophil gelatinase associated lipocalin; HO-1, heme oxygenase-1; SCr, serum creatinine; BUN, blood urea nitrogen; KDIGO, Kidney Disease: Improving Global Outcomes; ARF, acute renal failure; CPB, cardiopulmonary bypass.
Data Sharing Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of General Hospital of Ningxia Medical University (No. KYLL-2022-0589). This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding
Supported by Science and Technology Research Project of Ningxia University in 2022 (NYG2022047).
Disclosure
The authors declare that they have no competing interests.
References
1. Zhuo L, Xu Y, Zhang C, et al. 完全胸腔镜下微创心脏瓣膜手术的研究进展 [Research progress of complete thoracoscopic minimally invasive heart valve surgery]. Guoji Xinxueguanbing Zazhi. 2021;48(04):210–213. Chinese.
2. Vives N, Wijeysundera D, Marczin N, et al. Cardiac surgery associated acute kidney injury. Interact Cardiovasc Thorac Surg. 2014;18(5):637–645. doi:10.1093/icvts/ivu014
3. Nina VJ, Matias MM, Brito DJ, et al. Acute kidney injury after coronary artery bypass grafting: assessment using RIFLE and AKIN criteria. Rev Bras Cir Cardiovasc. 2013;28(2):231–237. doi:10.5935/1678-9741.20130033
4. Mao H, Katz N, Ariyanon W, et al. Cardiac surgery-associated acute kidney injury. Cardiorenal Med. 2013;3(3):178–199. doi:10.1159/000353134
5. Zhao HX, Zhao J, Li J, et al. 尿 NGAL、NAG及KIM-1联合检测在高龄老年急性肾损伤早期诊断中的价值 [Value of urine NGAL, NAG and KIM-1 combined detection in early diagnosis of acute kidney injury in elderly patients]. Guoji Mininaoxitong Zazhi. 2020;40(2):285–289. Chinese.
6. Ling GX, Luo C, Li YG, et al. Advances in acute kidney injury after cardiac surgery. Medical Review. 2018;24(6):1103–1108.
7. Haase M, Bellomo R, Devarajan P, et al. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54(6):1012–1024. doi:10.1053/j.ajkd.2009.07.020
8. Philippidis P, Mason JC, Evans BJ, et al. Hemoglobin scavenger receptor CD 163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinfl ammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res. 2004;94(1):119–126. doi:10.1161/01.RES.0000109414.78907.F9
9. KHWAJA A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. doi:10.1159/000339789
10. Yin G, Chen X. 急性肾损伤的定义及其临床诊断新标准 [Definition of acute kidney injury and new criteria for clinical diagnosis]. Xibu Yixue. 2013;25(12):1916–1917. Chinese.
11. Dabbagh A, Esmailian F, Aranki S. Translated by Zhang Peide. Postoperative Intensive Care in Adult Cardiac Surgery.
12. Han WK, Waikar SS, Johnson A, et al. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008;73(7):863–869. doi:10.1038/sj.ki.5002715
13. Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Ephrol. 2003;14(10):2534–2543. doi:10.1097/01.ASN.0000088027.54400.C6
14. Devarajan P. Emerging biomarkers of acute kidney injury. Contrib Nephrol. 2007;156:203–212.
15. Bennett M, Dent CL, Ma Q, et al. Urine NGAL predicts severity of acute kidney injury aft er cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3(3):665–673. doi:10.2215/CJN.04010907
16. Kidher E, Harling L, Ashrafian H, et al. Pulse wave velocity and neutrophil gelatinase-associated lipocalin as predictors of acute kidney injury following aortic valve replacement. J Cardiothorac Surg. 2014;9(1):89. doi:10.1186/1749-8090-9-89
17. Wang Q, Luo WJ, Zhou QL. NGAL和HO-1在瓣膜置换术致急性肾损伤中的临床意义及干预研究 [Clinical significance and intervention of NGAL and HO-1 in patients with acute kidney injury induced by valve replacement]. Zhongnan Daxue Xuebao. 2014;30(10):1001–1006. Chinese.
18. Billings FT, Yu C, Byrne JG, et al. Heme oxygenase-1 and acute kidney injury following cardiac surgery. Cardiorenal Med. 2014;4(1):12–21. doi:10.1159/000357871