Scientists from the University of Wisconsin–Madison in the US have coaxed light-sensitive eye cells grown in a lab to reconnect after separation, an important step for transplantation into patients to treat various eye diseases.
Working together, these photoreceptor cells combine with other cells to form the retina; a thin layer of tissue at the back of the eye responsible for transforming wavelengths of light into signals the brain interprets as vision.
It’s been a goal of researchers to grow retinal cells outside the body and use them to replace dead or dysfunctional tissues inside the eye.
In 2014, the researchers generated organoids (cell clusters self-organized into 3D forms in the lab) that resembled the form and function of a real retina. They did this by reprogramming human skin cells to act as stem cells, which were then encouraged to develop into several types of retinal cell.
Last year, the same team published studies showing that lab-grown retinal cells could respond to different wavelengths and intensities of light, as well as reach out towards neighboring cells to make connections.
According to lead researcher ophthalmologist David Gamm, this new study is “the last piece of the puzzle”.
“We wanted to use the cells from those organoids as replacement parts for the same types of cells that have been lost in the course of retinal diseases,” says Gamm.
“But after being grown in a laboratory dish for months as compact clusters, the question remained – will the cells behave appropriately after we tease them apart? Because that is key to introducing them into a patient’s eye.”
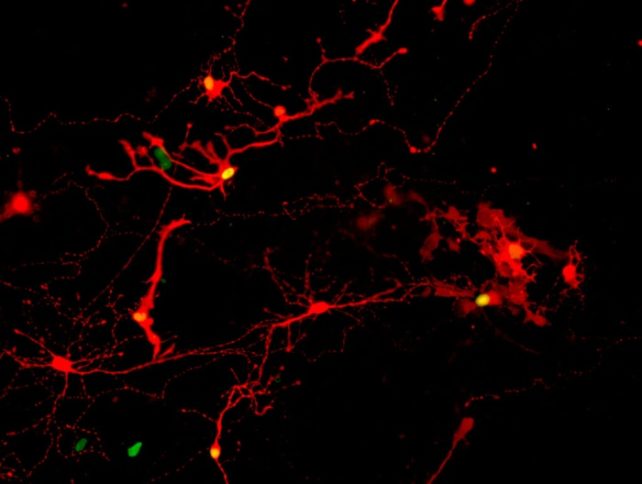
That functionality depends on cells being able to connect with one another using extensions called axons, with a chemical signal-box called a synapse forming a junction.
Seeing axons stretching between cells is one thing. To ensure working connections had been made, the team pulled clusters of retinal cells apart and watched them reconnect.
A rabies virus was then added, which was seen migrating between the retinal cells over the course of a week, indicating that synaptic connections had indeed been made.
“We’ve been quilting this story together in the lab, one piece at a time, to build confidence that we’re headed in the right direction,” says Gamm, from the University of Wisconsin-Madison.
“It’s all leading, ultimately, to human clinical trials, which are the clear next step.”
Further analysis revealed that the cell types that were most commonly forming synapses were the photoreceptors, commonly distinguished as rods and cones. That’s encouraging, because these cell types are the ones lost in diseases such as retinitis pigmentosa and age-related macular degeneration.
There was also evidence of cell types called retinal ganglion cells forming synapses. Replacing these cells in the eye could be useful in treating disorders such as glaucoma, where the optic nerve connecting the eye to the brain becomes damaged.
“That was an important revelation for us,” says Gamm. “It really shows the potentially broad impact these retinal organoids could have.”
The research has been published in PNAS.





